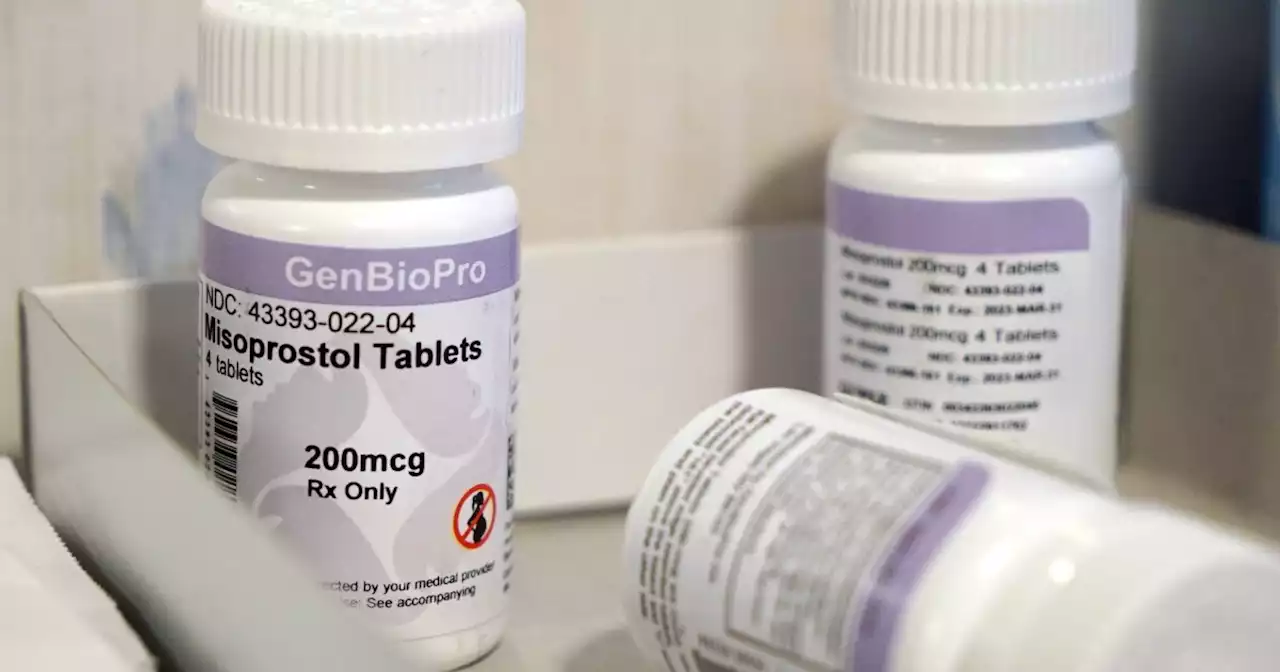
[This is part three of a series on the first-of-its-kind federal lawsuit, Americans for Hippocratic Medicine v. U.S. Food and Drug Administration, in which doctors and medical practitioners are asking the courts to hold the FDA accountable for its reckless and illegal approval of dangerous chemical…
n 2000, the Food and Drug Administration failed in its duty to protect the American public with its initial approval of chemical abortion drugs. But in the decades since, it has added insult to injury. Presented with two clear opportunities to correct course and protect American women and girls from these dangerous drugs, the FDA chose, both times, to ignore the evidence and silence the voices of concerned citizens.
In 2002, two professional medical associations filed a citizen petition, challenging the FDA’s initial approval and asking the agency to complete the studies it had neglected to perform, particularly the pediatric studies. The petition included dozens of pages of testimony and evidence of the dangers of chemical abortion.
Finally, in 2016, after nearly a decade and a half of silence, the FDA denied the citizen petition in a flurry of hand-waving. On the very same day, as a slap in the face to the many women who had been harmed by chemical abortion drugs since 2000, the FDA approved major and dangerous changes to the chemical abortion drug regimen in response to a request from Danco Laboratories, the corporation that manufactures chemical abortion drugs.
The FDA did all this despite the fact that chemical abortions have a complication rate of four times that of surgical abortions. It ignored the reality that at least 20% of women who use chemical abortion drugs will have to seek medical help afterward. In fact, the FDA facilitated the burial of those women’s stories. As part of the major changes, the FDA eliminated the requirement for healthcare workers to report nonfatal adverse effects from chemical abortion drugs.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Rising Concern Over Red Dye No. 3Consumer Reports petitions FDA to prohibit its use in food, dietary supplements, and ingested drugs.
Rising Concern Over Red Dye No. 3Consumer Reports petitions FDA to prohibit its use in food, dietary supplements, and ingested drugs.
Read more »
 FDA approves world-first brain modulation device to treat PTSDGrayMatters Health has announced that it has received clearance from the US Food and Drug Administration to market its healing product Prism, conceived for treating post-traumatic stress disorder.
FDA approves world-first brain modulation device to treat PTSDGrayMatters Health has announced that it has received clearance from the US Food and Drug Administration to market its healing product Prism, conceived for treating post-traumatic stress disorder.
Read more »
 FDA proposes using salt substitutes in more foodsAs part of the Biden administration's National Strategy on Hunger, Nutrition and Health, the U.S. Food and Drug Administration will propose an update on more use of salt substitutes.
FDA proposes using salt substitutes in more foodsAs part of the Biden administration's National Strategy on Hunger, Nutrition and Health, the U.S. Food and Drug Administration will propose an update on more use of salt substitutes.
Read more »
 FDA gives 2nd safety nod to cultivated meat, produced without slaughtering animalsGOOD Meat, which grows chicken and other meat from animal cells in a production facility, is the second company to cross this hurdle. The move brings no-kill meat closer to sale in the U.S.
FDA gives 2nd safety nod to cultivated meat, produced without slaughtering animalsGOOD Meat, which grows chicken and other meat from animal cells in a production facility, is the second company to cross this hurdle. The move brings no-kill meat closer to sale in the U.S.
Read more »
 ‘Lessons have not been learned’: FDA knew of positive test months before latest infant formula recallIn late February, formula giant Reckitt issued a press release recalling 145,000 cans of Enfamil ProSobee Simply Plant-Based Infant Formula over the “possibility of cross-contamination with Cronobacter sakazakii”
‘Lessons have not been learned’: FDA knew of positive test months before latest infant formula recallIn late February, formula giant Reckitt issued a press release recalling 145,000 cans of Enfamil ProSobee Simply Plant-Based Infant Formula over the “possibility of cross-contamination with Cronobacter sakazakii”
Read more »
 FDA Approves Pharming's Immune Disorder DrugThe US Food and Drug Administration on Friday approved Pharming Group's drug to treat a rare genetic disorder that leads to a weakened immune system, the Dutch company said.
FDA Approves Pharming's Immune Disorder DrugThe US Food and Drug Administration on Friday approved Pharming Group's drug to treat a rare genetic disorder that leads to a weakened immune system, the Dutch company said.
Read more »