The FDA is authorizing a second omicron-targeted bivalent COVID-19 booster vaccine for people over 65 or who are immunocompromised, the agency announces.
they offer stronger protection against infection and serious illness from omicron than the original shot.
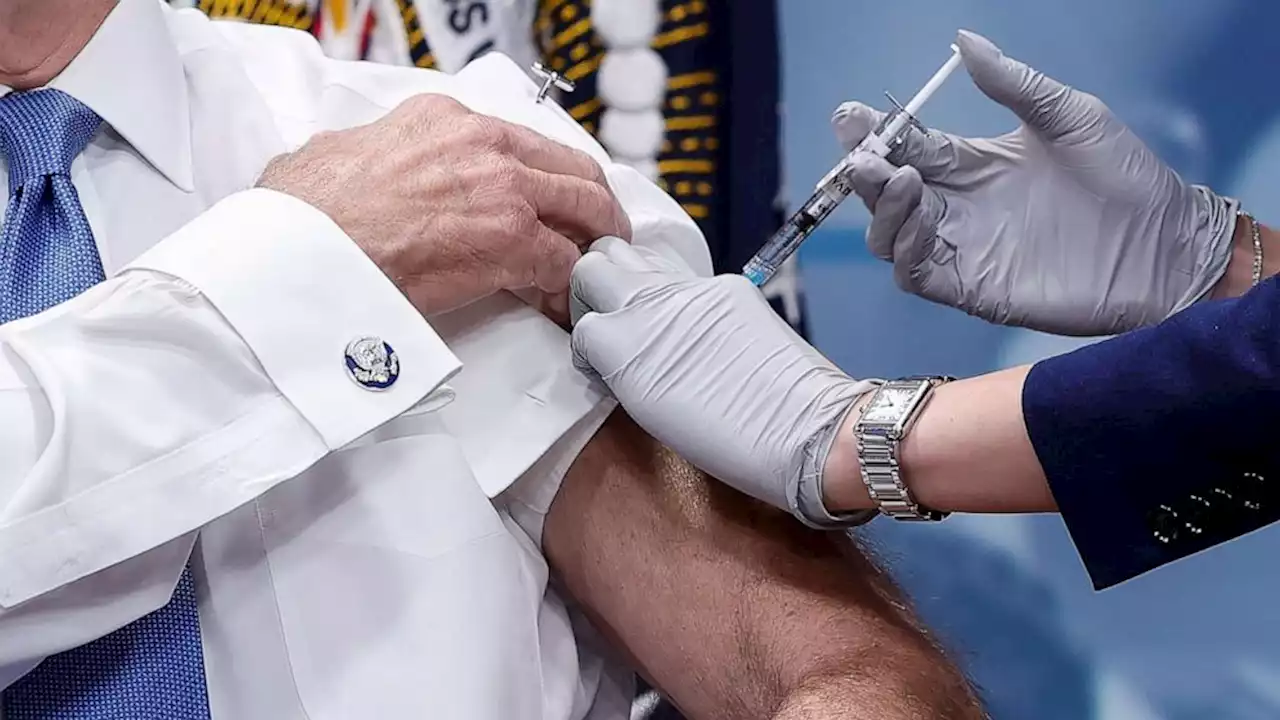
President Joe Biden receives his updated COVID-19 booster in the South Court Auditorium at the White House campus on Oct. 25, 2022, in Washington, D.C.Now, they'll be the only vaccines available in the US. Adults who haven't been vaccinated and who want to get vaccinated can get just a single dose of that vaccine, rather than a series of multiple shots. Unvaccinated children will still get multiple shots.
Vaccine uptake has slowed to a crawl in the U.S. Only 16.7% of people in the US overall and less than half of people over 65 have received a bivalent booster shot, according to data from the Centers for Disease Control and Prevention. Even though FDA is making a second bivalent booster shot available to people over 65, it's not clear how widely it'll be used.
But a single, annual shot could help encourage more vaccination, says ABC News contributor Dr. John Brownstein, an epidemiologist and chief innovation officer at Boston Children's Hospital"Simple and clear messaging is critical when it comes to ensuring vaccine uptake," he said. "Moving to a model that resembles the yearly flu shot campaign will help focus messaging and help increase population immunity ahead of a potential fall surge.
"We still have to recognize that the future of covid remains uncertain and this trial vaccination strategy may have to be modified," he says.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
Read more »
 FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
Read more »
 FDA clears second bivalent COVID boosters for some AmericansSome Americans will soon be able to get another shot of the bivalent COVID-19 vaccines, the FDA authorized Tuesday, including seniors ages 65 and older and people with compromised immune systems.
FDA clears second bivalent COVID boosters for some AmericansSome Americans will soon be able to get another shot of the bivalent COVID-19 vaccines, the FDA authorized Tuesday, including seniors ages 65 and older and people with compromised immune systems.
Read more »
 FDA authorizes 2nd dose of updated Covid booster for older adultsPeople 65 and up can get a second dose of the updated booster at least four months after their last dose.
FDA authorizes 2nd dose of updated Covid booster for older adultsPeople 65 and up can get a second dose of the updated booster at least four months after their last dose.
Read more »
 FDA authorizes additional omicron Covid shots for seniors and people with weak immune systemsAlthough the burden of the pandemic has eased substantially, the CDC says Covid continues to kill more than 1,300 people per week.
FDA authorizes additional omicron Covid shots for seniors and people with weak immune systemsAlthough the burden of the pandemic has eased substantially, the CDC says Covid continues to kill more than 1,300 people per week.
Read more »
 Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Read more »