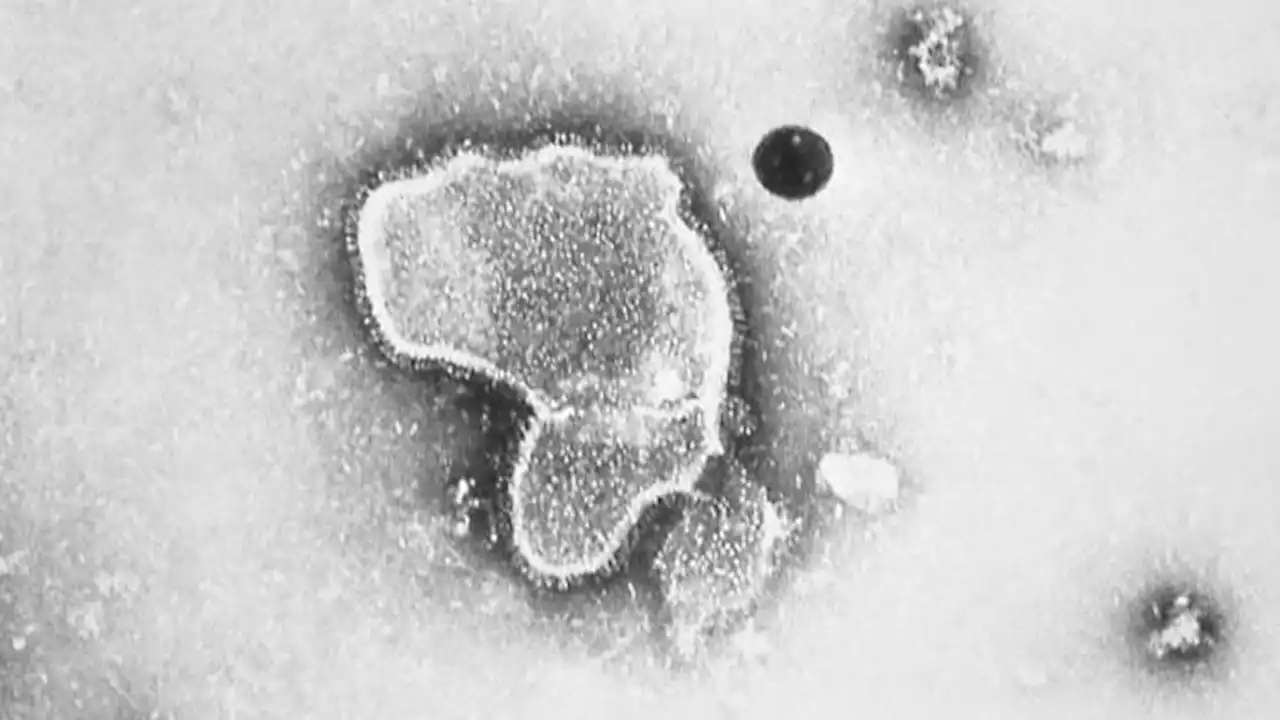
The FDA sees a potential risk and has asked Pfizer to conduct a safety study on Guillain-Barre after a potential approval, which the company said it would do.
Eli Lilly recruits Black patients for Alzheimer trial as drugmakers seek more diversity in clinical studies
"Given the temporal association and biological plausibility, FDA agrees with the assessments of the investigators that these events were possibly related to study vaccine," the agency said. But GSK, in its briefing document, said a Guillain-Barre diagnosis was not confirmed due to the absence of exam results and because there was no information on whether alternative causes were investigated. The patient's case was considered resolved after six months, the company said.The Centers for Disease Control and Prevention's committee of independent vaccine advisors grappled with the three cases of Guillain-Barre syndrome during a meeting open to the public Thursday. Dr.
Although Pfizer and GSK have asked the FDA to approve their respective vaccines for people ages 60 and older, the CDC workgroup generally favored a recommendation for seniors ages 65 and older. The CDC advisory committee did not vote on any recommendations for the RSV vaccines this week.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer/BioNTech apply for full FDA approval of updated COVID vaccinePfizer Inc and its German partner BioNTech SE said on Friday they filed an application to the U.S Food and Drug Administration (FDA) for a full approval of their Omicron-adapted COVID-19 vaccine.
Pfizer/BioNTech apply for full FDA approval of updated COVID vaccinePfizer Inc and its German partner BioNTech SE said on Friday they filed an application to the U.S Food and Drug Administration (FDA) for a full approval of their Omicron-adapted COVID-19 vaccine.
Read more »
 FDA Broadens Warning on Potentially Contaminated Eye Products🚫 Do not purchase or use Delsam Pharma’s Artificial Eye Ointment, the FDA warns.
FDA Broadens Warning on Potentially Contaminated Eye Products🚫 Do not purchase or use Delsam Pharma’s Artificial Eye Ointment, the FDA warns.
Read more »
 FDA releases draft guidance on how plant-based milk items should be labeled | CNNThe US Food and Drug Administration issued draft guidance Wednesday on how companies should label plant-based products that are marketed and sold as alternatives to dairy milk, such as almond milk, oat milk or soy milk.
FDA releases draft guidance on how plant-based milk items should be labeled | CNNThe US Food and Drug Administration issued draft guidance Wednesday on how companies should label plant-based products that are marketed and sold as alternatives to dairy milk, such as almond milk, oat milk or soy milk.
Read more »
 Soy, oat, almond, other dairy alternatives can be called milk, FDA proposesDraft U.S. rules would allow soy, oat, almond and other drinks that bill themselves as “milk' to keep using the name.
Soy, oat, almond, other dairy alternatives can be called milk, FDA proposesDraft U.S. rules would allow soy, oat, almond and other drinks that bill themselves as “milk' to keep using the name.
Read more »
 No cow needed: Oat and soy can be called milk, FDA proposesFood and Drug Administration officials issued guidance that says plant-based beverages don’t pretend to be from dairy animals – and that U.S. consumers aren’t confused by the difference.
No cow needed: Oat and soy can be called milk, FDA proposesFood and Drug Administration officials issued guidance that says plant-based beverages don’t pretend to be from dairy animals – and that U.S. consumers aren’t confused by the difference.
Read more »
 No cow needed: Oat and soy can be called milk, FDA proposesSoy, oat, almond and other drinks that bill themselves as “milk” can keep using the name, according to draft federal rules released Wednesday. Food and Drug Administration officials issued guidance that says plant-based beverages don’t pretend to be from dairy animals – and that U.S.
No cow needed: Oat and soy can be called milk, FDA proposesSoy, oat, almond and other drinks that bill themselves as “milk” can keep using the name, according to draft federal rules released Wednesday. Food and Drug Administration officials issued guidance that says plant-based beverages don’t pretend to be from dairy animals – and that U.S.
Read more »