U.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
WASHINGTON — U.S. regulators on Thursday strictly limited who can receive Johnson & Johnson’s COVID-19 vaccine due to the ongoing risk of rare but serious blood clots.
“If there’s an alternative that appears to be equally effective in preventing severe outcomes from COVID-19, we’d rather see people opting for that,” Marks said. “But we’ve been careful to say that-- compared to no vaccine-- this is still a better option.” As of mid-March, federal scientists had identified 60 cases of the side effect, including nine that were fatal. That amounts to 3.23 blood clot cases per 1 million J&J shots. The problem is more common in women under 50, where the death rate was roughly 1 per million shots, according to Marks.
Under the new FDA instructions, J&J’s vaccine could still be given to people who had a severe allergic reaction to one of the other vaccines and can’t receive an additional dose. J&J’s shot could also be an option for people who refuse to receive the mRNA vaccines from Pfizer and Moderna, and therefore would otherwise remain unvaccinated, the agency said.
U.S. regulators decided the benefits of J&J’s one-and-done vaccine outweighed what was considered a very rare riCOVID-19 causes deadly blood clots, too. But the vaccine-linked kind is different, believed to form because of a rogue immune reaction to the J&J and AstraZeneca vaccines because of how they’re made. Clots form in unusual places, such as veins that drain blood from the brain, and in patients who also develop abnormally low levels of the platelets that form clots.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA restricts J&J's COVID-19 vaccine due to blood clot riskThe Food and Drug Administration said the shot should only be given to adults who cannot receive a different vaccine or specifically request J&J's vaccine.
FDA restricts J&J's COVID-19 vaccine due to blood clot riskThe Food and Drug Administration said the shot should only be given to adults who cannot receive a different vaccine or specifically request J&J's vaccine.
Read more »
 FDA restricts J&J’s COVID-19 vaccine due to blood clot riskFDA officials said in a statement that they decided to restrict J&J’s vaccine after taking another look at data on the risk of life-threatening blood clots within two weeks of vaccination.
FDA restricts J&J’s COVID-19 vaccine due to blood clot riskFDA officials said in a statement that they decided to restrict J&J’s vaccine after taking another look at data on the risk of life-threatening blood clots within two weeks of vaccination.
Read more »
 FDA restricts J&J’s COVID-19 vaccine due to blood clot riskU.S. regulators on Thursday strictly limited who can receive Johnson & Johnson’s COVID-19 vaccine due to the ongoing risk of rare but serious blood clots.
FDA restricts J&J’s COVID-19 vaccine due to blood clot riskU.S. regulators on Thursday strictly limited who can receive Johnson & Johnson’s COVID-19 vaccine due to the ongoing risk of rare but serious blood clots.
Read more »
 FDA restricts J&J’s COVID-19 vaccine due to risk of blood clotsThe U.S. Food and Drug Administration announced on Thursday that it was limiting the authorized use of the Johnson & Johnson COVID-19 vaccine for adults due to studies showing people developing rare and potentially life-threatening blood clots.
FDA restricts J&J’s COVID-19 vaccine due to risk of blood clotsThe U.S. Food and Drug Administration announced on Thursday that it was limiting the authorized use of the Johnson & Johnson COVID-19 vaccine for adults due to studies showing people developing rare and potentially life-threatening blood clots.
Read more »
 J&J COVID-19 vaccine distribution restricted due to blood clot risk, FDA saysU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
J&J COVID-19 vaccine distribution restricted due to blood clot risk, FDA saysU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
Read more »
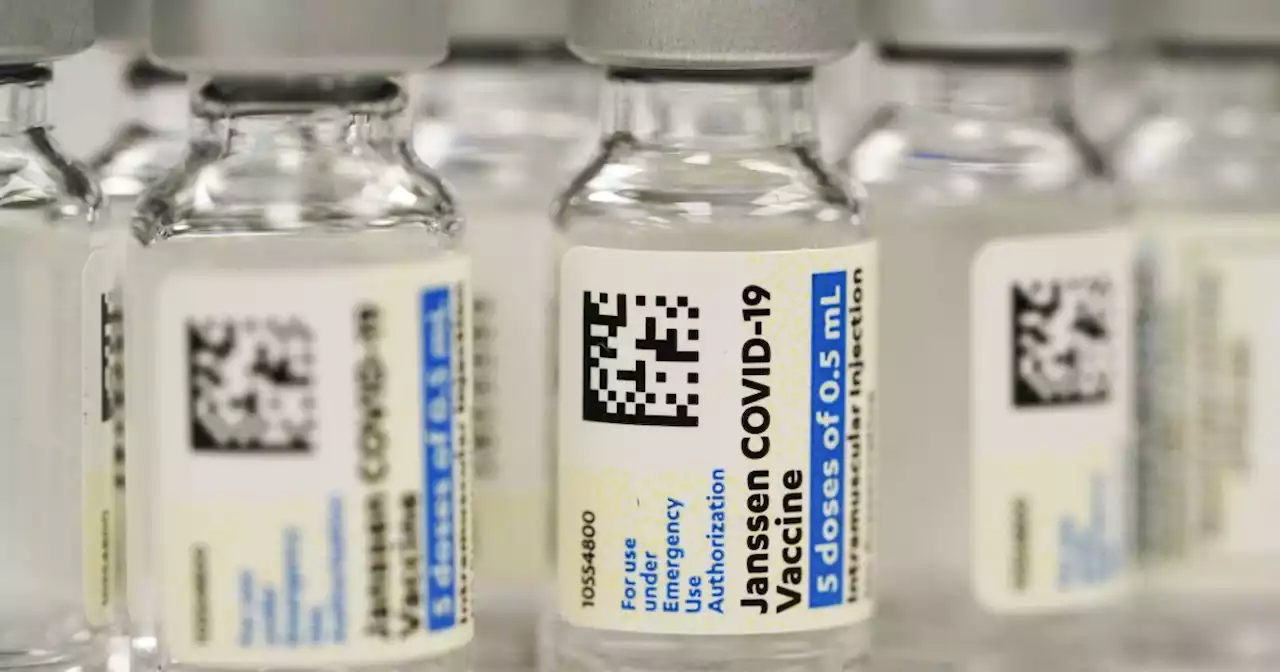 FDA restricts Johnson & Johnson's COVID-19 vaccine due to blood clot riskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
FDA restricts Johnson & Johnson's COVID-19 vaccine due to blood clot riskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
Read more »
