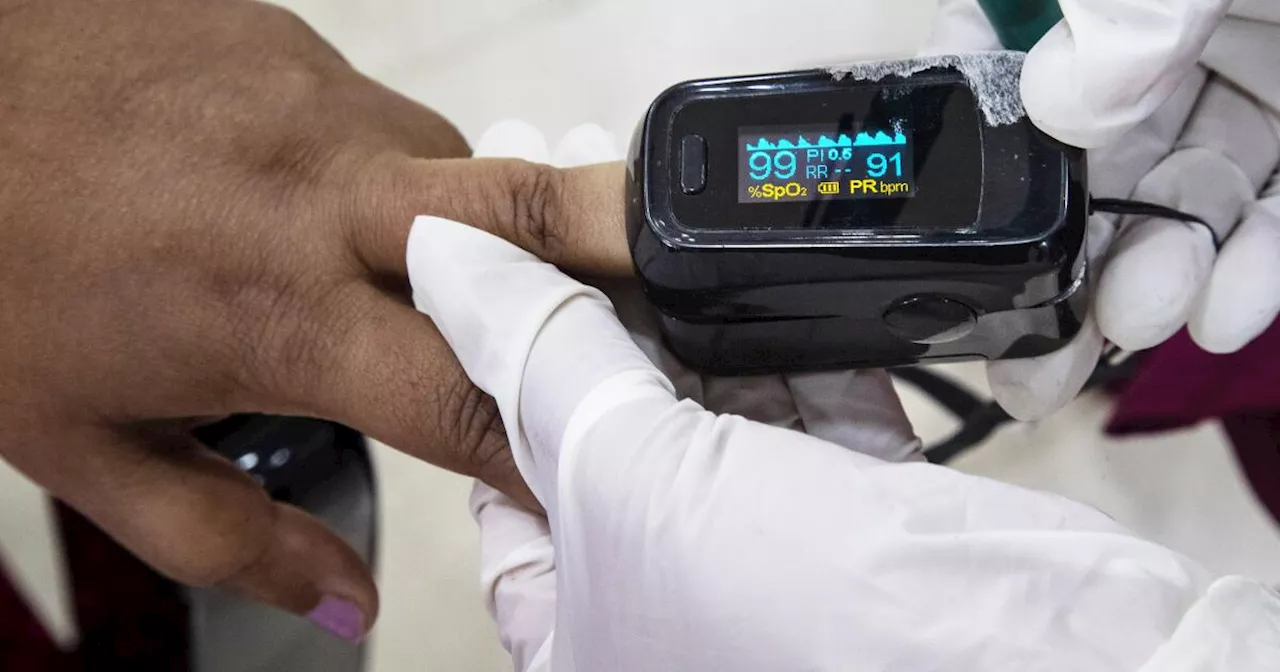
The FDA is proposing new rules requiring manufacturers of pulse oximeters to collect more data to ensure their devices are accurate for people with dark skin. This comes after studies have shown potential inaccuracies in readings for patients with darker pigmentation.
Manufacturers of medical devices that rapidly measure blood oxygen levels would have to collect additional data to demonstrate that their products work for people with dark skin, according to a new proposal from US authorities published on Monday. The recommendations from the Food and Drug Administration ( FDA ) apply to pulse oximeters, which are devices that are placed on the fingers and used in hospitals and medical clinics to ensure that patients have enough oxygen in their blood.
The FDA wants companies to conduct more extensive studies and include more patients from different racial groups. By placing a device on a finger and then sending two wavelengths of light to the skin, the oximeter measures how much light is absorbed and estimates how much oxygen flows through the blood. Oximeters were a critical part of emergency care for COVID-19 patients during the pandemic. However, several studies have suggested that darker skin pigmentation can sometimes affect the accuracy of readings. In 2021, the FDA warned doctors about potential inaccuracies with oximeters after a study found that the devices tended to overestimate the oxygen levels of Black patients, which could lead to delays in treatment and increase the risk of death. The problem has become an example of the potential racial biases in medical technology, leading to multiple meetings and studies by FDA regulators since 2022
FDA Oximeters Blood Oxygen Skin Pigmentation Medical Technology
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
Read more »
 FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
Read more »
 FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos. The rule was proposed Thursday and is intended to reassure consumers about the safety of makeup, baby powder and other personal care products.
FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos. The rule was proposed Thursday and is intended to reassure consumers about the safety of makeup, baby powder and other personal care products.
Read more »
 FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos.
Read more »
 FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos
FDA proposes new testing rules to ensure cosmetics are asbestos-freeThe Food and Drug Administration is proposing a rule that cosmetic companies would have to take extra steps to ensure that any products containing talc are free of asbestos
Read more »
 FDA Proposes New Rules for Pulse Oximeters to Improve Accuracy for Patients of ColorThe FDA is proposing new rules that would require companies making pulse oximeters to conduct larger studies with more diverse patient populations. This comes after studies suggested that darker skin pigmentation can sometimes lead to inaccurate readings from these devices, which are crucial for monitoring oxygen levels in patients.
FDA Proposes New Rules for Pulse Oximeters to Improve Accuracy for Patients of ColorThe FDA is proposing new rules that would require companies making pulse oximeters to conduct larger studies with more diverse patient populations. This comes after studies suggested that darker skin pigmentation can sometimes lead to inaccurate readings from these devices, which are crucial for monitoring oxygen levels in patients.
Read more »