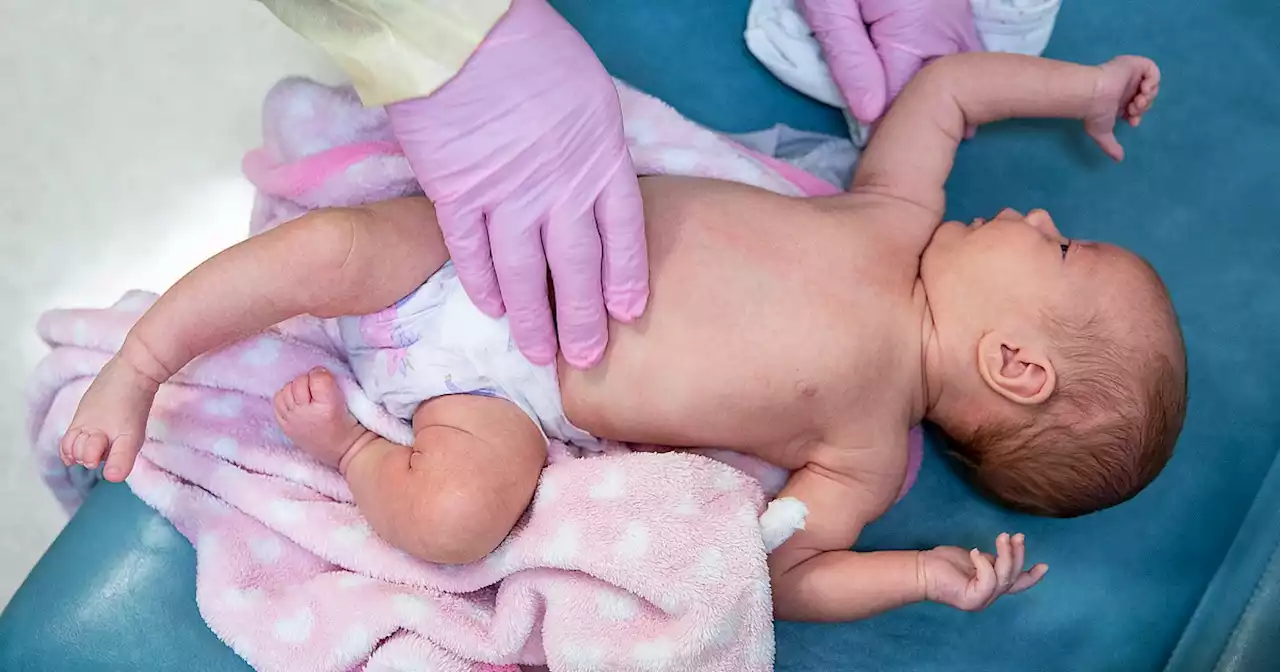
If eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
An independent advisory committee to the Food and Drug Administration will decide on Thursday whether to recommend an RSV vaccine for infants.
The shot the FDA committee is evaluating on Thursday would be given to pregnant people at 24 to 36 weeks’ gestation, and the protective antibodies transfer to infants through the placenta.In a clinical trial with nearly 7,400 participants, the vaccine lowered the risk of severe disease from RSV among infants by 82% within roughly three months after birth. By around six months, that efficacy was around 69%.
However, the agency noted that there was a slightly higher rate of preterm births — defined as before 37 weeks’ gestation — among people who received the vaccine versus those who got a placebo . That difference wasn’t statistically significant, though, so it’s unclear whether it was vaccine-related.in the general population: around 10%, according to the Centers for Disease Control and Prevention.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer RSV Vaccine for Infants Has ‘Generally Favorable' Safety Data, FDA Staff SayThe FDA is slated to make a decision on whether to approve Pfizer’s shot in August before respiratory syncytial virus season in the fall.
Pfizer RSV Vaccine for Infants Has ‘Generally Favorable' Safety Data, FDA Staff SayThe FDA is slated to make a decision on whether to approve Pfizer’s shot in August before respiratory syncytial virus season in the fall.
Read more »
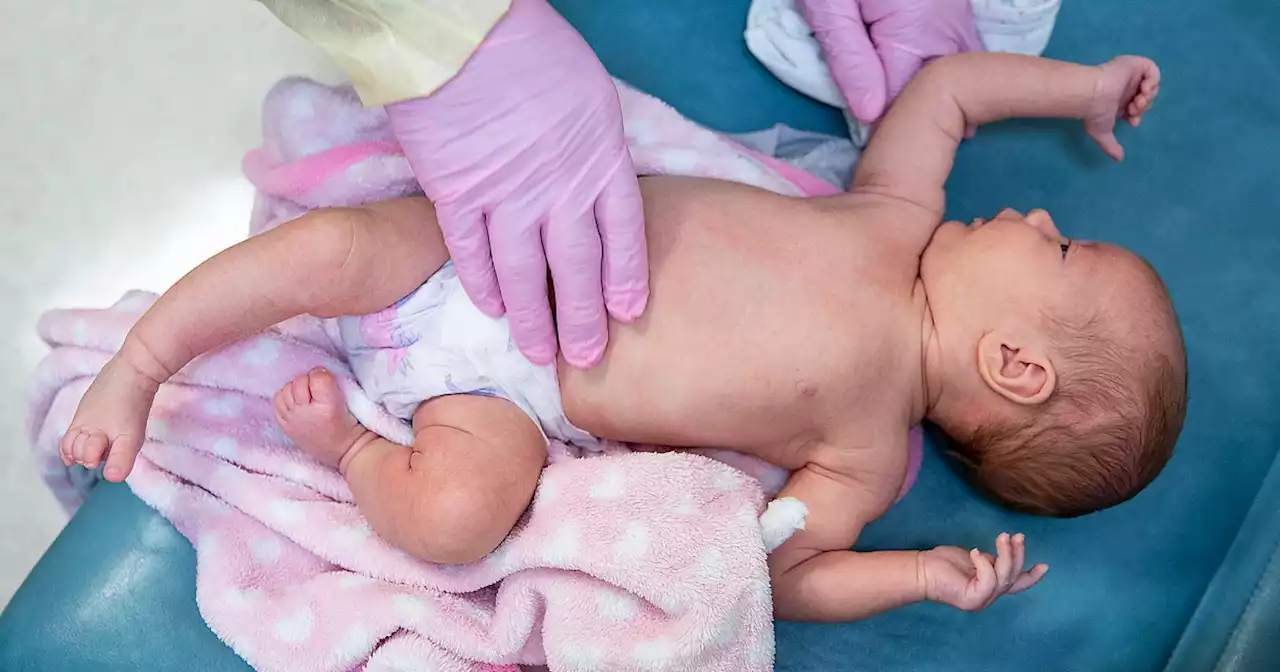 FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
Read more »
 Episode 1: RSV Trends After COVID-19: Back With a Vengeance📹 Join Drs Forest Arnold and Victoria Statler as they discuss RSV after the COVID pandemic. Tune in to hear about an increase in hospitalization rates, surprising seasonal shifts, and more. MedTwitter
Episode 1: RSV Trends After COVID-19: Back With a Vengeance📹 Join Drs Forest Arnold and Victoria Statler as they discuss RSV after the COVID pandemic. Tune in to hear about an increase in hospitalization rates, surprising seasonal shifts, and more. MedTwitter
Read more »
 Thermometers recalled due to reports of ‘severe skin burns’: FDAThe health body is urging anyone handling the device to be careful with chemical leakage, as that could result in burns.
Thermometers recalled due to reports of ‘severe skin burns’: FDAThe health body is urging anyone handling the device to be careful with chemical leakage, as that could result in burns.
Read more »
 Menopause hot flashes medication receives FDA approvalThe once-a-day pill from Astellas Pharma uses a new approach, targeting brain connections that help control body temperature to treat moderate-to-severe symptoms, which can include sweating, flushing and chill.
Menopause hot flashes medication receives FDA approvalThe once-a-day pill from Astellas Pharma uses a new approach, targeting brain connections that help control body temperature to treat moderate-to-severe symptoms, which can include sweating, flushing and chill.
Read more »
 New HER2 Drug for Breast Cancer Stalled by FDAThe FDA said it needs more data the new antibody-drug conjugate (ADC) trastuzumab duocarmazine for use in advanced or metastatic breast cancer.
New HER2 Drug for Breast Cancer Stalled by FDAThe FDA said it needs more data the new antibody-drug conjugate (ADC) trastuzumab duocarmazine for use in advanced or metastatic breast cancer.
Read more »