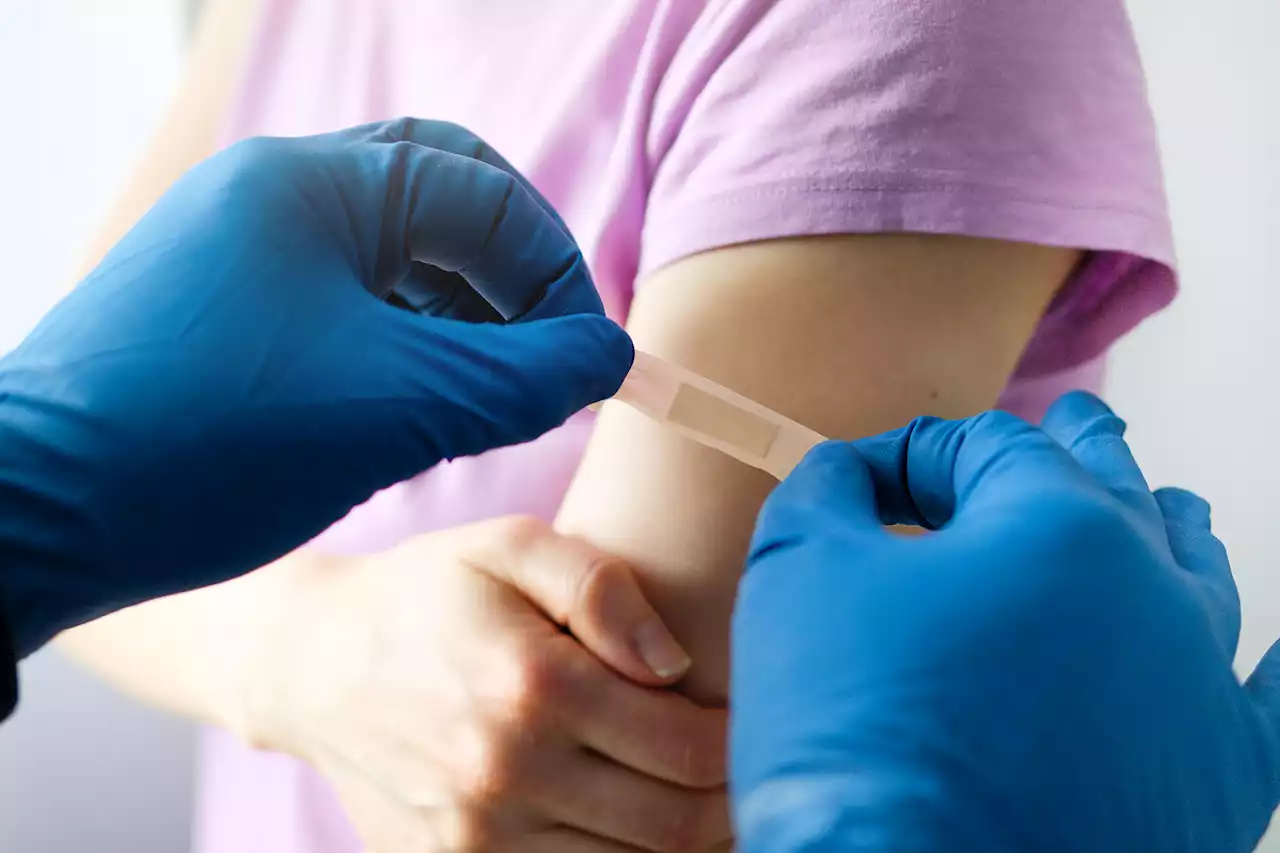
If the CDC also recommends the shots, everyone six months and older will be able to get a COVID-19 vaccine
, more than 2 million cases of COVID-19 have occurred among children 4 years old or younger since the start of the pandemic, leading to more than 440 deaths.
and offer some freedom to families that have remained largely isolated until their youngest members could be vaccinated.The Pfizer-BioNTech vaccine for kids ages 6 months to 4 years old is a three-shot regimen, and each dose is one-tenth the dosage given to adults. Children would receive two doses three weeks apart, and a third dose at least two months later.
until the companies provided additional information on whether adding a third dose would increase levels of virus-fighting antibodies, and therefore immunity, against COVID-19. The company reported those data in May.Moderna’s vaccine for children ages 6 months through 5 years old is given in two shots, and each dose is one-quarter the dose of that used in adults. Those immunized with Moderna would get two doses spaced four weeks apart.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Read more »
 FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
Read more »
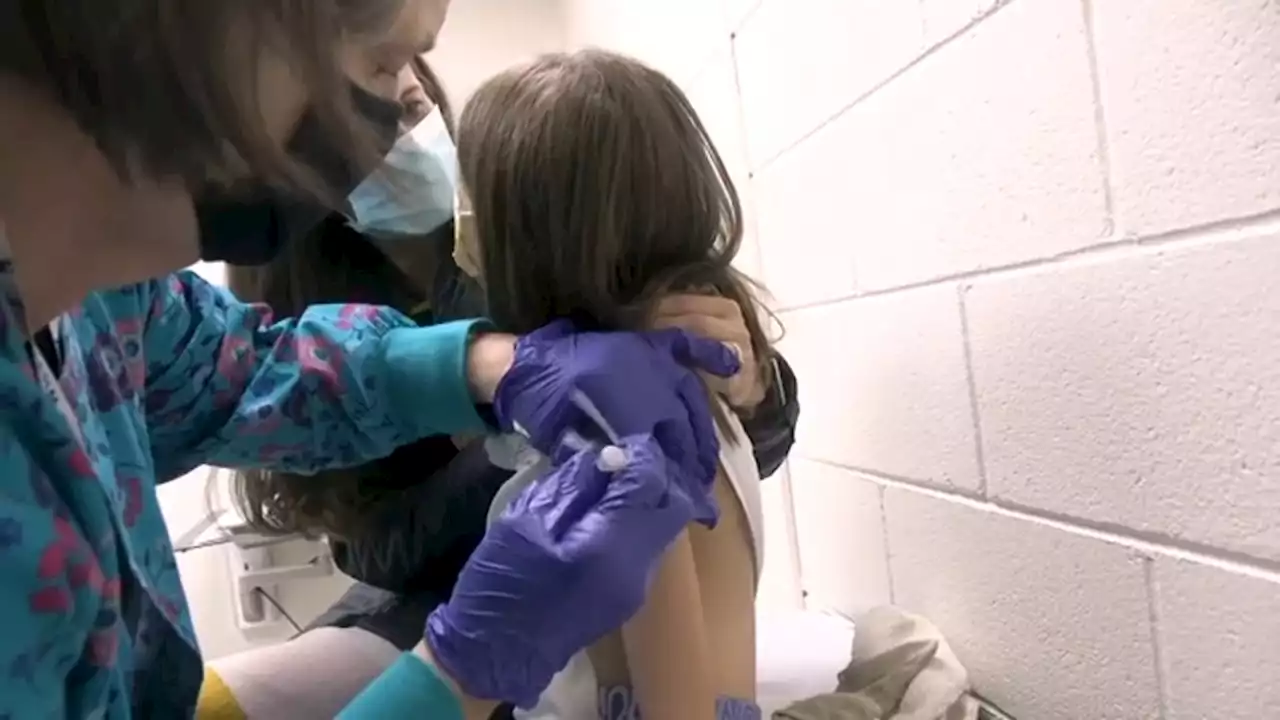 FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
Read more »
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Read more »