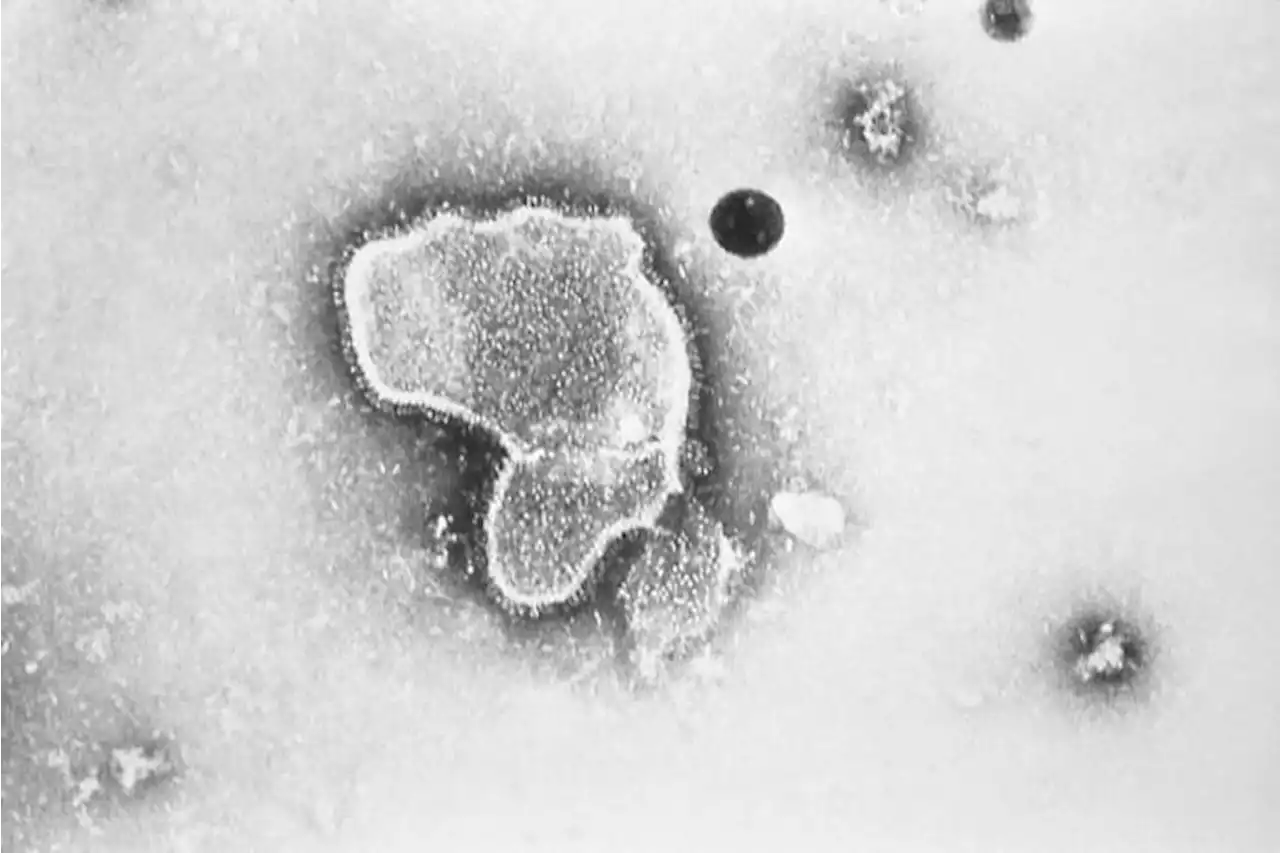
Federal health advisers have narrowly backed an experimental vaccine from Pfizer that could become the first shot to protect older adults against the respiratory illness known as RSV. rsv pfizer kprc2 click2houston
FILE - This 1981 electron microscope image provided by the Centers for Disease Control and Prevention shows a human respiratory syncytial virus, also known as RSV. On Tuesday, Feb. 28, 2023, a panel of U.S. Food and Drug Administration advisers narrowly backed an experimental vaccine from Pfizer that could become the first shot to protect older adults against the RSV respiratory virus.
“I think the primary endpoint was clearly met here,” said Dr. Daniel Feikin, a respiratory disease consultant who voted in favor of the vaccine. “It’s disappointing we don’t have more data on the high-risk groups and severe outcomes.” For most healthy people, RSV is a cold-like nuisance. But for the very young, the elderly and people with certain health problems, it can be serious and even life-threatening. The virus can cause infections deep in the lungs, triggering pneumonia.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA advisors recommend Pfizer RSV vaccine for older adults, despite possible Guillain-Barre risks
FDA advisors recommend Pfizer RSV vaccine for older adults, despite possible Guillain-Barre risks
Read more »
 FDA panel narrowly backs Pfizer's RSV vaccine for older adultsFederal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against...
FDA panel narrowly backs Pfizer's RSV vaccine for older adultsFederal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against...
Read more »
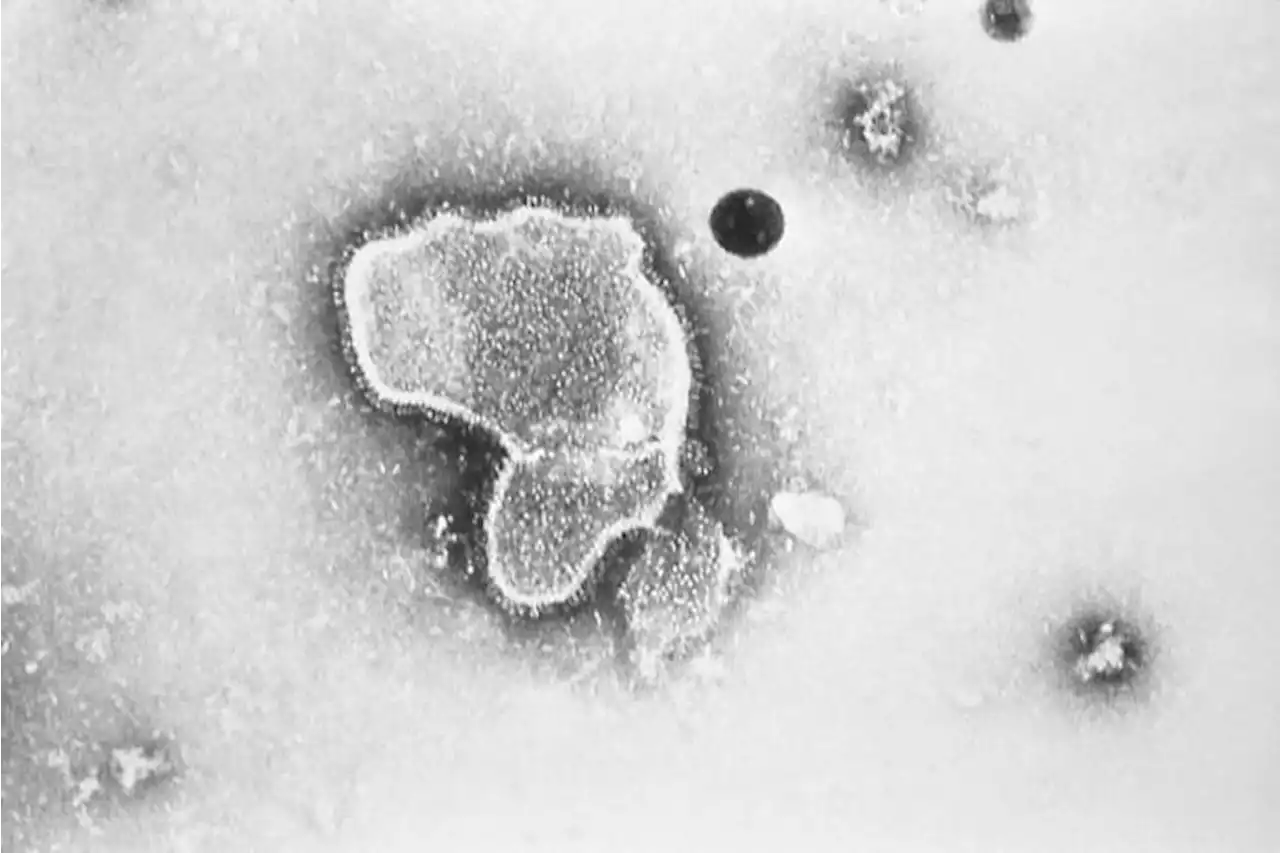 FDA panel narrowly backs Pfizer RSV vaccine for older adultsFederal health advisers have narrowly backed an experimental vaccine from Pfizer that could become the first shot to protect older adults against the respiratory illness known as RSV.
FDA panel narrowly backs Pfizer RSV vaccine for older adultsFederal health advisers have narrowly backed an experimental vaccine from Pfizer that could become the first shot to protect older adults against the respiratory illness known as RSV.
Read more »
 FDA warns of Guillain-Barre syndrome as possible risk of Pfizer’s RSV vaccineGuillain-Barre syndrome, or inflammatory neuropathy, is a disorder in which the body’s immune system mistakenly attacks the nerves.
FDA warns of Guillain-Barre syndrome as possible risk of Pfizer’s RSV vaccineGuillain-Barre syndrome, or inflammatory neuropathy, is a disorder in which the body’s immune system mistakenly attacks the nerves.
Read more »
 Pfizer, GSK face FDA panel review in race for RSV vaccinesThe frontrunners in a crowded race to develop the first respiratory syncytial virus (RSV) vaccine - Pfizer Inc and GSK - will face scrutiny from a panel of experts to the U.S. Food and Drug Administration this week.
Pfizer, GSK face FDA panel review in race for RSV vaccinesThe frontrunners in a crowded race to develop the first respiratory syncytial virus (RSV) vaccine - Pfizer Inc and GSK - will face scrutiny from a panel of experts to the U.S. Food and Drug Administration this week.
Read more »
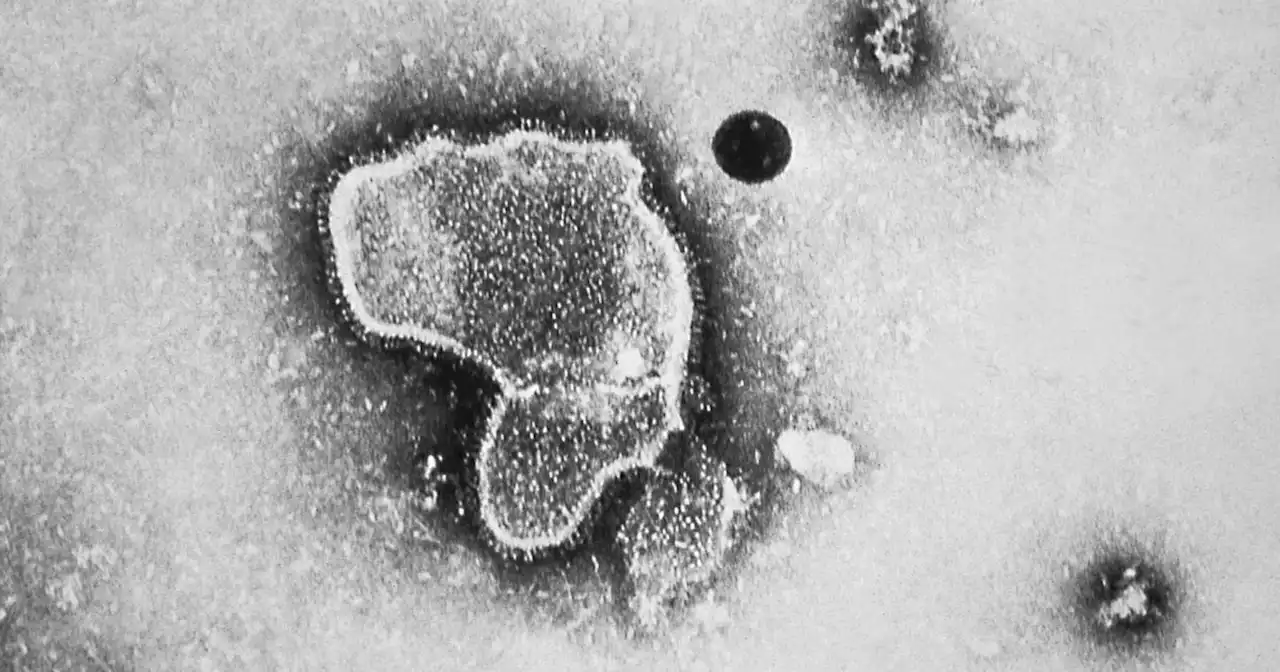 Paving the way for the world's first RSV vaccine, FDA advisers recommend shot from PfizerBREAKING: FDA advisers recommend that the agency approve the country’s first RSV vaccine for older people, a shot from Pfizer for adults ages 60 and up.
Paving the way for the world's first RSV vaccine, FDA advisers recommend shot from PfizerBREAKING: FDA advisers recommend that the agency approve the country’s first RSV vaccine for older people, a shot from Pfizer for adults ages 60 and up.
Read more »