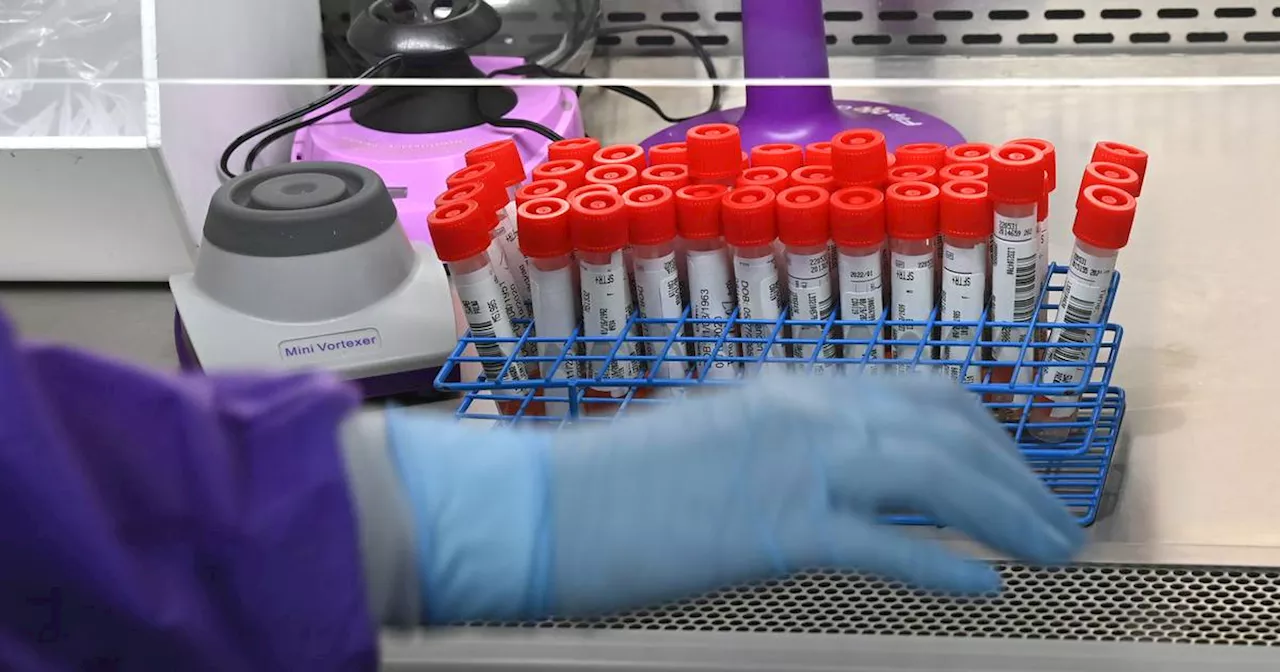
The FDA has finalized a divisive plan to regulate some lab tests, including those used to diagnose cancer and Alzheimer’s, over concerns about reliability and risk to patients.
By Rachel Roubein, Daniel Gilbert, The Washington PostThe Food and Drug Administration is seeking to regulate laboratory developed tests.
“The agency cannot stand by while Americans continue to rely on results of these tests without assurance that they work,” FDA Commissioner Robert M. Califf said in a news release. The FDA does not review most lab tests before patients use them. But it finalized a plan to phase in the regulation of lab tests over the course of four years. Many tests will now have to go through premarket review from the FDA as well as adhere to other requirements, like the reporting of adverse events.
“However, we remain concerned that many vital tests developed in hospitals and health systems will still get caught up in unnecessary and costly paperwork,” Stacey Hughes, an executive vice president with the American Hospital Association, said in a statement. Decades ago, LDTs were much less common. But the prevalence of such tests has grown substantially with the ability to ship samples of patients’ blood and saliva to labs around the country, the FDA said.
The issue has been in the crosshairs of lawmakers on Capitol Hill for several years. Congress has failed to pass bipartisan legislation solidifying the agency’s power. The lack of guidance prompted the agency to issue its own rules.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
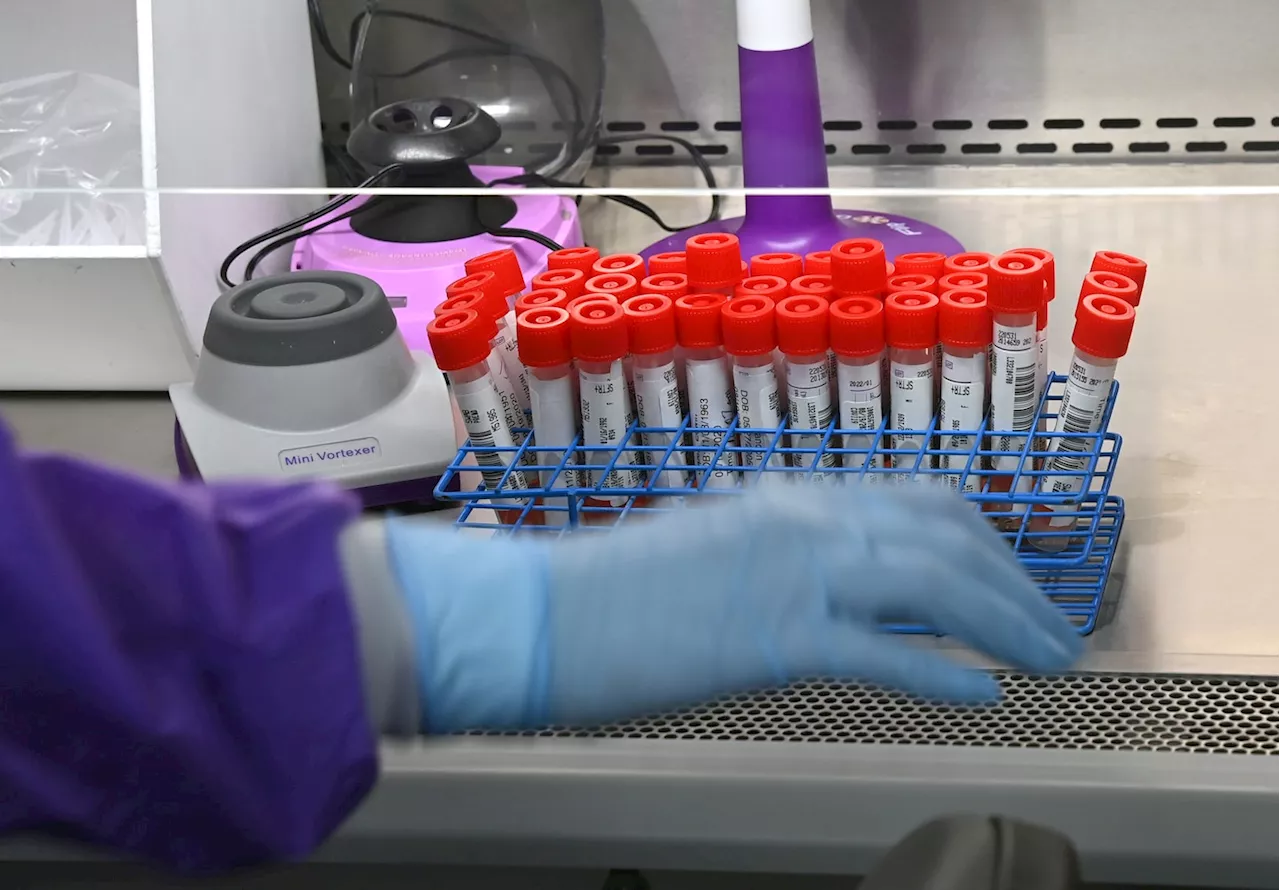 FDA moves to regulate some tests it says may be unreliableThe FDA has finalized a divisive plan to regulate some lab tests, including those used to diagnose cancer and Alzheimer’s disease, over concerns about reliability and risk to patients.
FDA moves to regulate some tests it says may be unreliableThe FDA has finalized a divisive plan to regulate some lab tests, including those used to diagnose cancer and Alzheimer’s disease, over concerns about reliability and risk to patients.
Read more »
 FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Food and Drug Administration has finalized a rule to regulate medical tests that have long escaped oversight.
FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Food and Drug Administration has finalized a rule to regulate medical tests that have long escaped oversight.
Read more »
 FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Associated Press
FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Associated Press
Read more »
 FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Food and Drug Administration has finalized a rule to regulate medical tests that have long escaped oversight
FDA brings lab tests under federal oversight in bid to improve accuracy and safetyThe Food and Drug Administration has finalized a rule to regulate medical tests that have long escaped oversight
Read more »
 Bird Flu: Why FDA Says Milk Safe Despite 1 in 5 Positive Tests for VirusTwo human cases of bird flu have been confirmed so far, with one likely resulting from exposure to dairy cows.
Bird Flu: Why FDA Says Milk Safe Despite 1 in 5 Positive Tests for VirusTwo human cases of bird flu have been confirmed so far, with one likely resulting from exposure to dairy cows.
Read more »
 FDA increases control over laboratory-developed tests after COVID-19 fiascoThe FDA is now classifying LDTs as medical devices. This follows criticism following the FDA not approving COVID-19 rapid tests more quickly in 2020.
FDA increases control over laboratory-developed tests after COVID-19 fiascoThe FDA is now classifying LDTs as medical devices. This follows criticism following the FDA not approving COVID-19 rapid tests more quickly in 2020.
Read more »