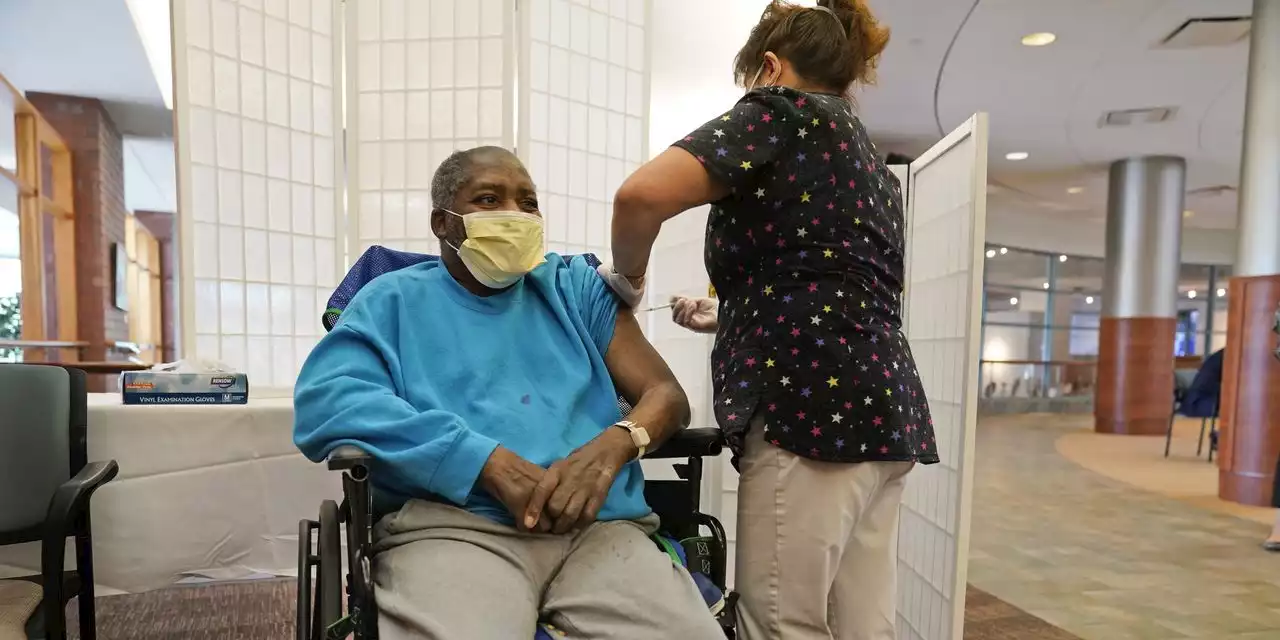
The FDA has fully approved Moderna's Covid-19 vaccine, as case numbers and hospitalizations in the U.S. continue to decline
The Food and Drug Administration on Monday gave its full approval to Moderna Inc.’s Covid-19 vaccine, branded Spikevax, for use in adults 18 and older, making it the second fully approved Covid-19 vaccine after one from Pfizer Inc. and BioNTech SE.
The agency granted full approval to Moderna’s shot after conducting a more thorough review of the evidence for the vaccine’s safety and efficacy.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Moderna's COVID-19 vaccine gets full FDA approvalModerna's COVID-19 vaccine called “Spikevax” has been given full approval from the U.S. Food and Drug Administration.
Read more »
 Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Moderna COVID-19 vaccine gets full FDA approvalThe Moderna Covid-19 vaccine received full approval from the U.S. Food and Drug administration Monday, 11 months after the shot was given an emergency use authorization.
Read more »
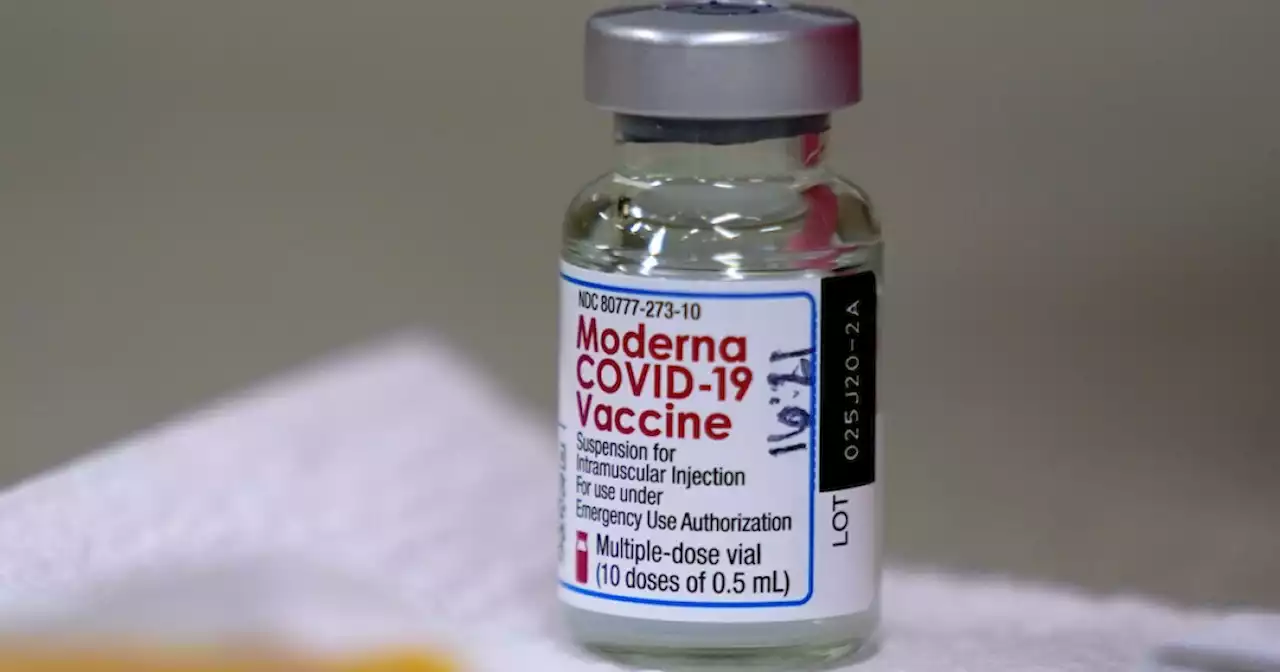 Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Moderna's COVID-19 vaccine granted full FDA approval, company saysModerna says the Food and Drug Administration has issued full approval for its COVID-19 vaccine for use in people aged 18 and older.
Read more »
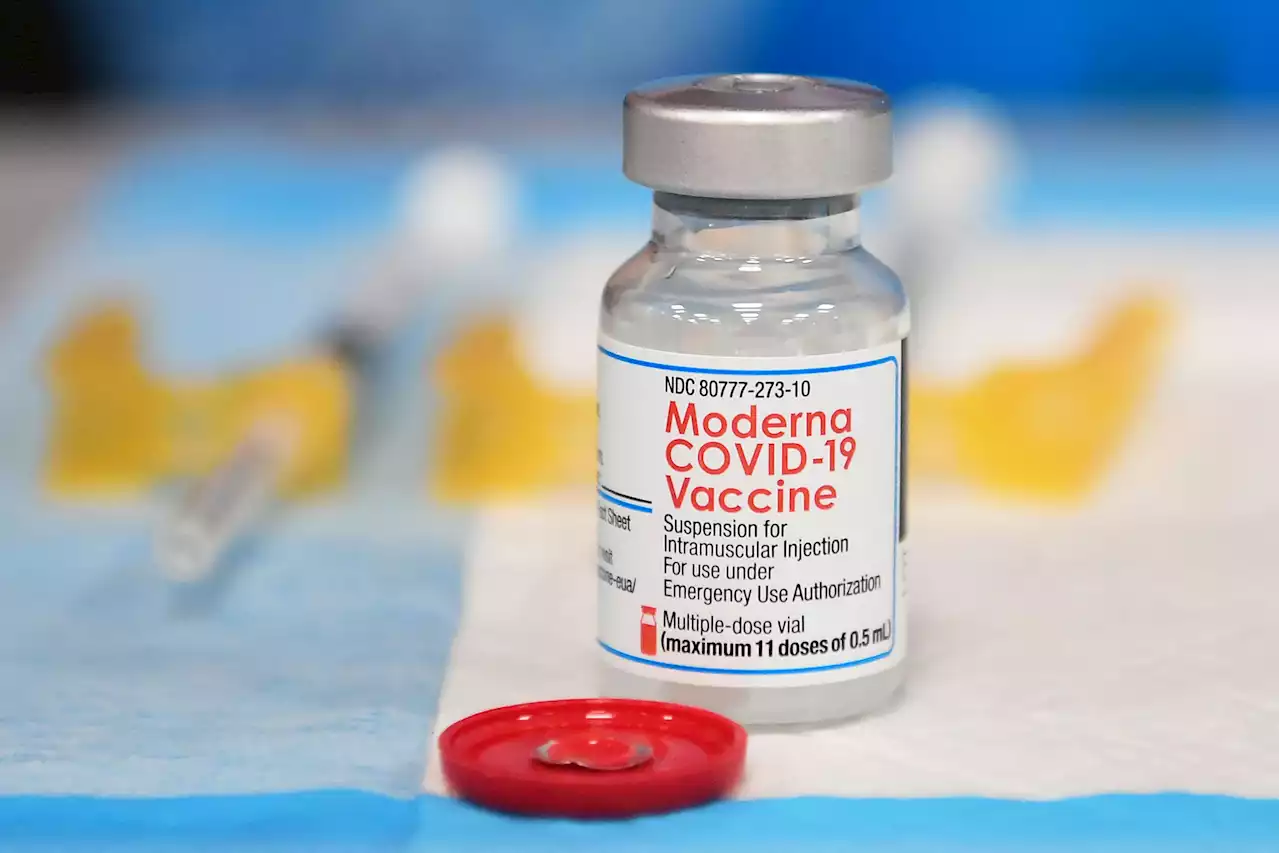 FDA Gives Moderna Full Approval for Its COVID-19 VaccineModerna says U.S. health regulators have given full approval to its COVID-19 vaccine after reviewing additional data on its safety and effectiveness
FDA Gives Moderna Full Approval for Its COVID-19 VaccineModerna says U.S. health regulators have given full approval to its COVID-19 vaccine after reviewing additional data on its safety and effectiveness
Read more »
 Moderna’s COVID-19 vaccine gets full FDA approvalIt’ll be marketed as Spikevax.
Moderna’s COVID-19 vaccine gets full FDA approvalIt’ll be marketed as Spikevax.
Read more »