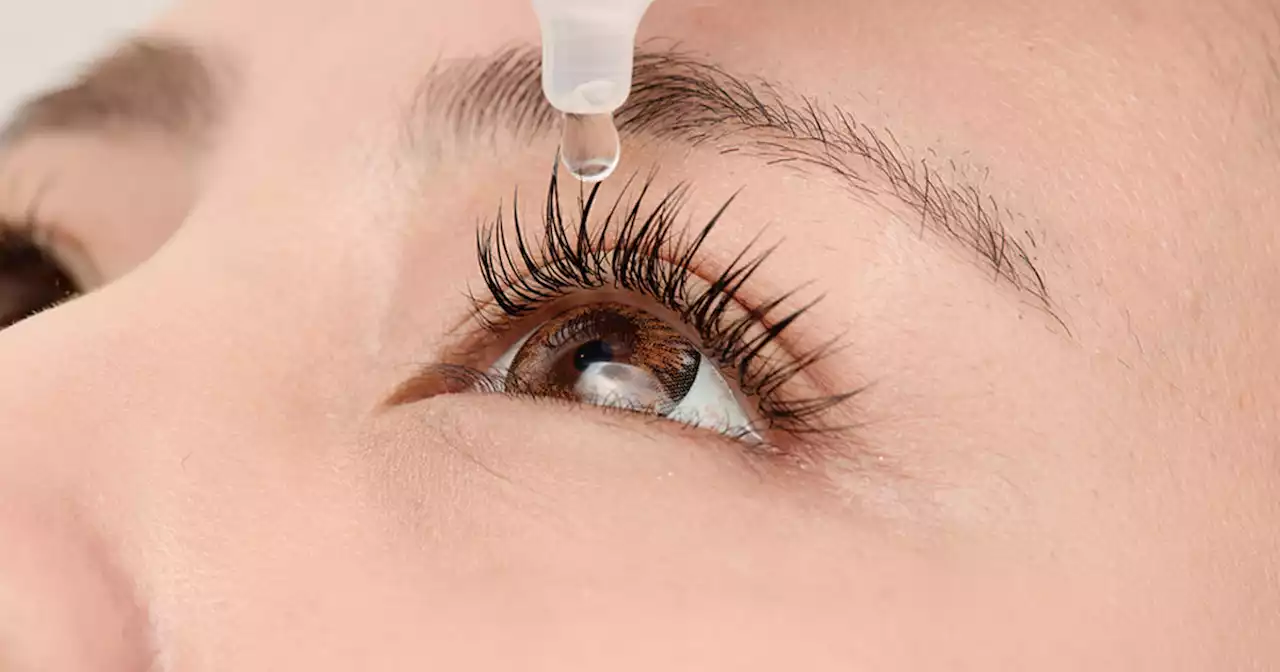
The FDA warned of another imported eye product that could be contaminated with bacteria, in the wake of an outbreak earlier this year of highly drug-resistant bacteria that hospitalized and blinded some patients.
58 patients have been identified. Since the alert earlier this month, a 13th state, Illinois, has been added to the list of jurisdictions with cases.
"We are actively gathering more information about long term patient outcomes, particularly for patients with eye infections," the spokesperson said. Most cases linked to the outbreak involved eye drops purchased online before the recall. However, one reported buying EzriCare at a Costco warehouse.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Broadens Warning on Potentially Contaminated Eye Products🚨RECALL ALERT:🚨 Do not purchase or use Delsam Pharma’s Artificial Eye Ointment, the FDA warns.
FDA Broadens Warning on Potentially Contaminated Eye Products🚨RECALL ALERT:🚨 Do not purchase or use Delsam Pharma’s Artificial Eye Ointment, the FDA warns.
Read more »
 FDA expands warning about potentially contaminated eye products amid outbreakThe FDA warned of another imported eye product that could be contaminated with bacteria, in the wake of an outbreak earlier this year of highly drug-resistant bacteria that hospitalized and blinded some patients.
FDA expands warning about potentially contaminated eye products amid outbreakThe FDA warned of another imported eye product that could be contaminated with bacteria, in the wake of an outbreak earlier this year of highly drug-resistant bacteria that hospitalized and blinded some patients.
Read more »
 Executive chairman of Spanish pharma company Grifols resignsSteven F. Mayer has resigned as executive chairman of the board of Spanish pharmaceutical company Grifols , the company said on Tuesday, citing health and other personal reasons.
Executive chairman of Spanish pharma company Grifols resignsSteven F. Mayer has resigned as executive chairman of the board of Spanish pharmaceutical company Grifols , the company said on Tuesday, citing health and other personal reasons.
Read more »
 Wyden: FDA Should Ignore Potential Court Order Blocking Abortion PillSen. Ron Wyden (D-OR) told the Biden administration that it should ignore an upcoming potential court order that would block the distribution of mifepristone, the first pill used in a two-step medication abortion regimen.
Wyden: FDA Should Ignore Potential Court Order Blocking Abortion PillSen. Ron Wyden (D-OR) told the Biden administration that it should ignore an upcoming potential court order that would block the distribution of mifepristone, the first pill used in a two-step medication abortion regimen.
Read more »
 FDA OKs Sparsentan for Rare Kidney DiseaseThe US FDA gave accelerated approval to sparsentan as the first non-immunosuppressive therapy labeled for IgA nephropathy. Conventional approval awaits clinical outcomes data.
FDA OKs Sparsentan for Rare Kidney DiseaseThe US FDA gave accelerated approval to sparsentan as the first non-immunosuppressive therapy labeled for IgA nephropathy. Conventional approval awaits clinical outcomes data.
Read more »
 Chemomab Therapeutics stock soars after FDA clears IND for SSc treatmentShares of Chemomab Therapeutics Ltd. shot up 46.9% in premarket trading Tuesday, after the Israel-based biotechnology company said the U.S. Food and Drug...
Chemomab Therapeutics stock soars after FDA clears IND for SSc treatmentShares of Chemomab Therapeutics Ltd. shot up 46.9% in premarket trading Tuesday, after the Israel-based biotechnology company said the U.S. Food and Drug...
Read more »