The FDA, which has taken a tougher stance on China-developed drugs, postponed a decision on one from Novartis and BeiGene for esophageal cancer because staff hadn’t completed required inspections in China
The Food and Drug Administration delayed a decision on a proposed new esophageal-cancer treatment from Novartis AG and BeiGene Ltd.
until the agency can complete inspections in China that have been delayed byThe drug, tislelizumab, was initially developed by BeiGene, a company with Chinese operations that is incorporated in the Cayman Islands. BeiGene first tested the drug in cancer patients in China, and the company has manufacturing operations there.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
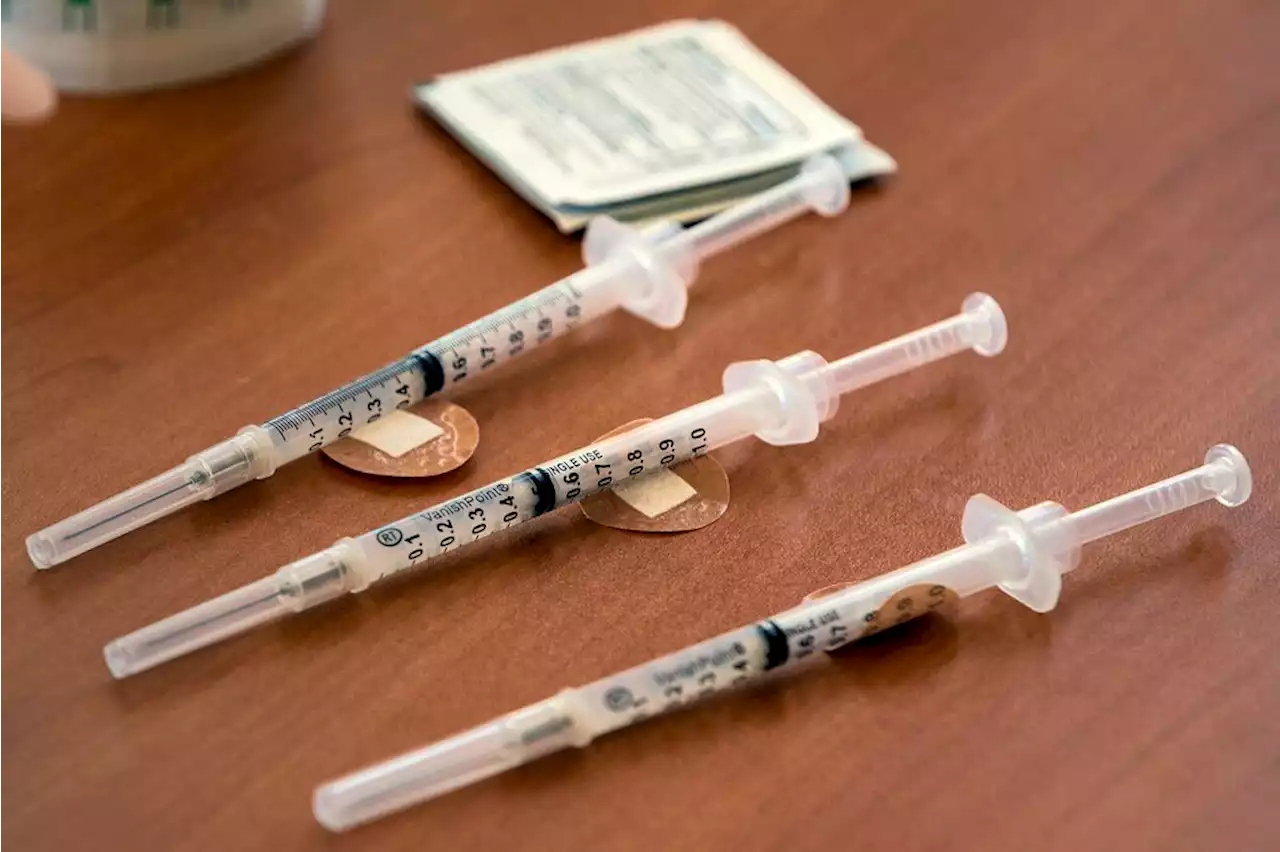 FDA working to allow boosters for all adultsSecond boosters are currently only authorized for people who are at least 50 years old or immunocompromised.
FDA working to allow boosters for all adultsSecond boosters are currently only authorized for people who are at least 50 years old or immunocompromised.
Read more »
 Honey-based sexual supplements may contain Cialis and Viagra ingredients, FDA warnsThe FDA sent warning letters to companies selling products with names like 'Royal Honey for Him' and 'X Rated Honey for Men' that may contain pharmaceuticals.
Honey-based sexual supplements may contain Cialis and Viagra ingredients, FDA warnsThe FDA sent warning letters to companies selling products with names like 'Royal Honey for Him' and 'X Rated Honey for Men' that may contain pharmaceuticals.
Read more »
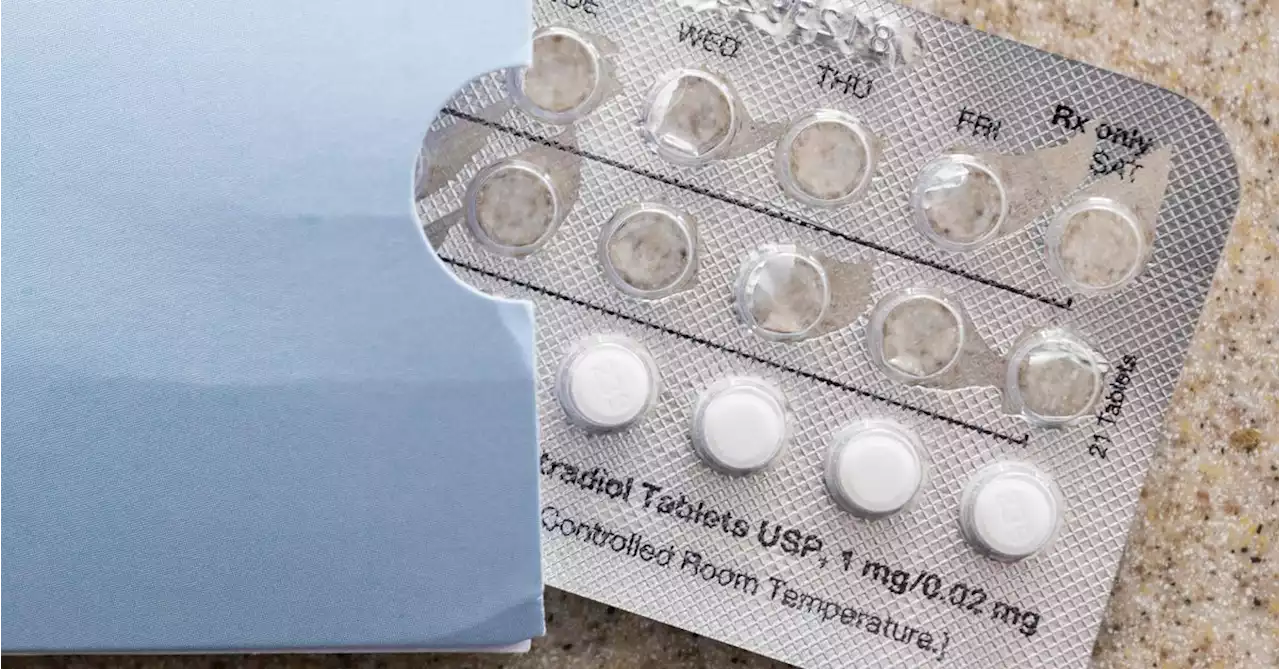 FDA to consider 'mini' over-the-counter birth control pillHRA Pharma makes a progestin-only 'mini pill.'
FDA to consider 'mini' over-the-counter birth control pillHRA Pharma makes a progestin-only 'mini pill.'
Read more »
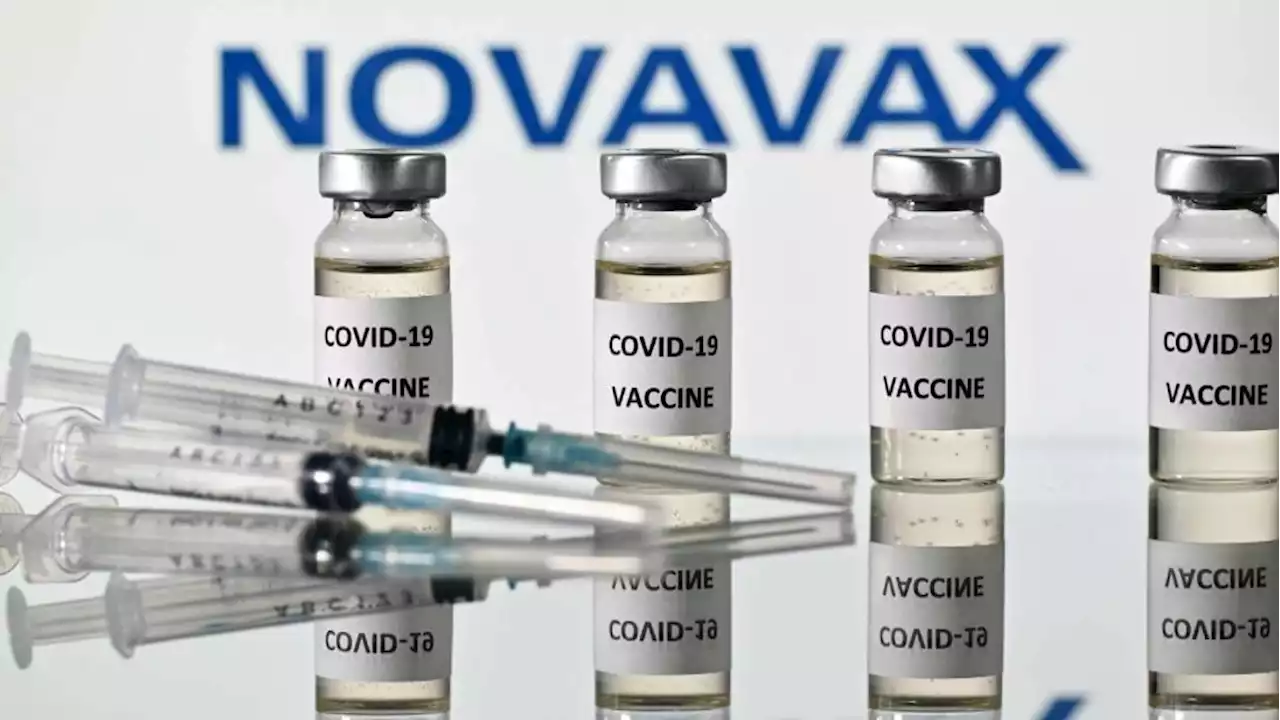 FDA gives emergency use authorization to Novavax's Covid-19 vaccineFDA gives emergency use authorization to Novavax's Covid-19 vaccine, which uses different technology than shots already available in US.
FDA gives emergency use authorization to Novavax's Covid-19 vaccineFDA gives emergency use authorization to Novavax's Covid-19 vaccine, which uses different technology than shots already available in US.
Read more »
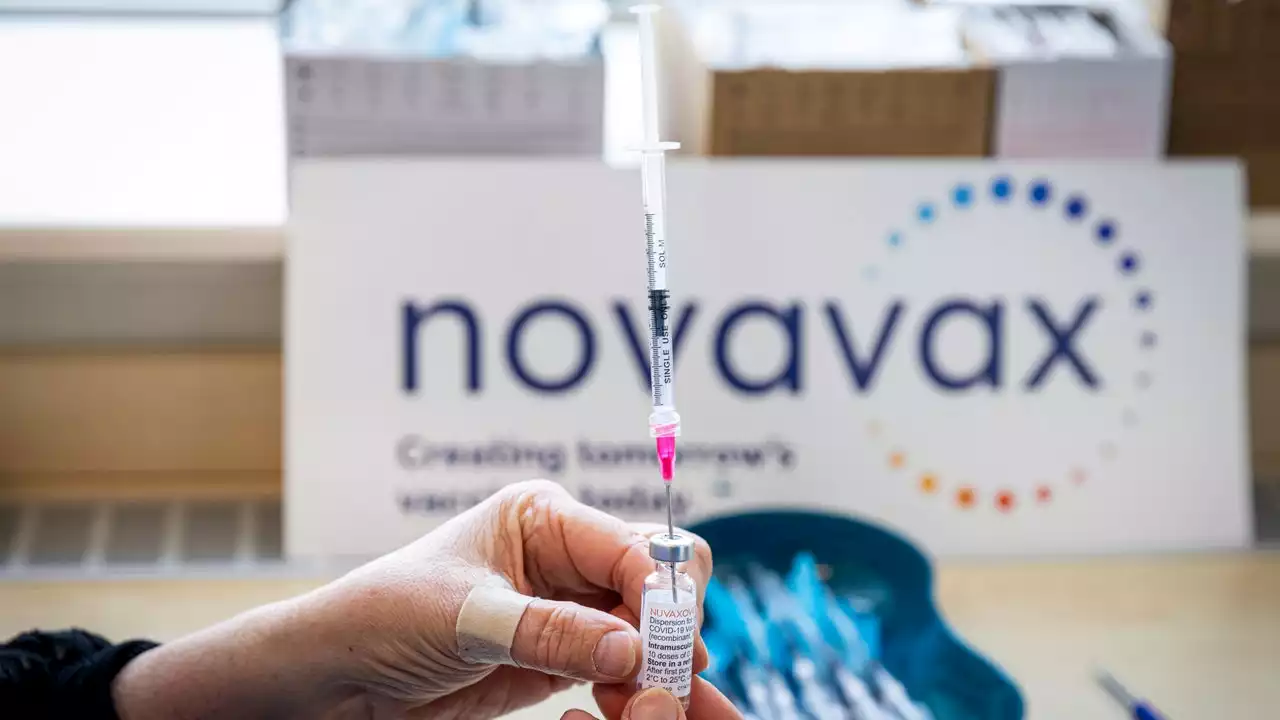 Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Read more »
 FDA Authorizes Novavax’s Covid-19 VaccineThe FDA has cleared use of Novavax’s Covid-19 vaccine, which uses an older technology that some people hesitant to get inoculated might be willing to get.
FDA Authorizes Novavax’s Covid-19 VaccineThe FDA has cleared use of Novavax’s Covid-19 vaccine, which uses an older technology that some people hesitant to get inoculated might be willing to get.
Read more »
