Up until now, the only real treatment has been a stem cell or bone marrow transplant. The new exa-cel treatment under FDA consideration can use the patient's own stem cells.
If approved, exa-cel would be the first FDA-approved treatment that uses genetic modification called CRISPR.sickle cell
The independent committee is helping the FDA think through how it should evaluate a treatment called exa-cel that could potentially cure people of sickle cell disease, a painful and deadly disease with no universally successful treatment. This was an ongoing discussion. There was no vote or decision about the therapy, but the discussion likely moves the US one step closer to approving a groundbreaking new treatment that uses gene editing.
Up until now, the only real treatment has been a stem cell or bone marrow transplant. For stem cells, fewer than 20% of patients have an appropriately matched donor, the FDA said, and the transplants are risky and may not work. Sometimes a transplant can kill the patient.The new exa-cel treatment under FDA consideration can use the patient's own stem cells.
An FDA presentation to the panel suggested the agency may have some questions about the data. It called a lack of confirmatory testing "concerning." It also noted the study's small patient size.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Read more »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Read more »
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Read more »
 FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
Read more »
 Crispr Therapeutics’ stock soars premarket after positive meeting of FDA panel on sickle-cell disease therapyCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
Crispr Therapeutics’ stock soars premarket after positive meeting of FDA panel on sickle-cell disease therapyCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
Read more »
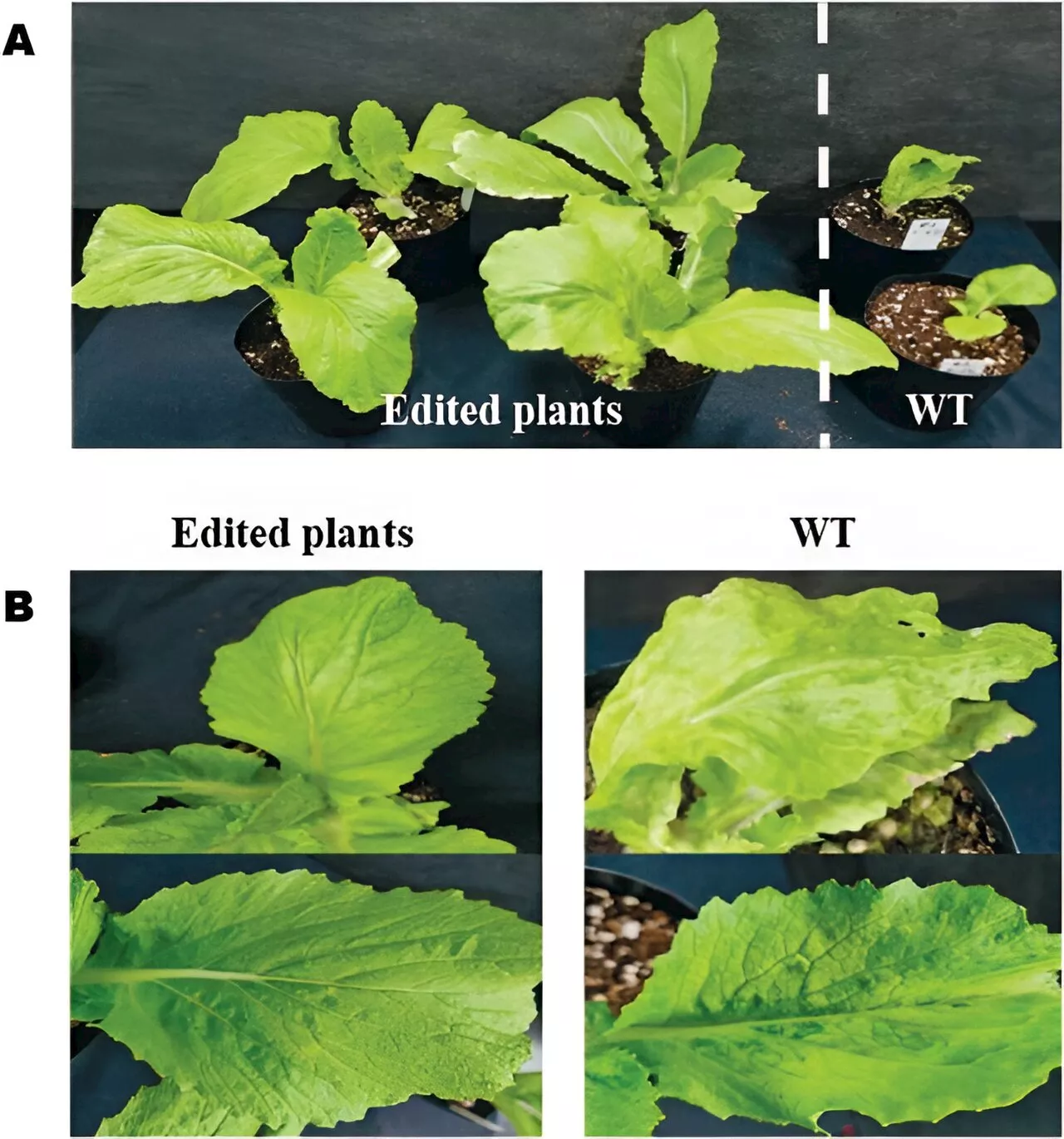 CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
Read more »
