Though the FDA and federal regulations call these actions recalls, they might be described more aptly as 'non-recalls.' And they have happened repeatedly in recent years.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure., the manufacturer explained,"Abbott is not removing product from commercial distribution." Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use. The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company's primary concern, and certain issues"can be safely and effectively remedied with a correction on site." In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of"destruction of the impeller blades," which could cause"low flow" and"embolization of the fractured impeller material," an
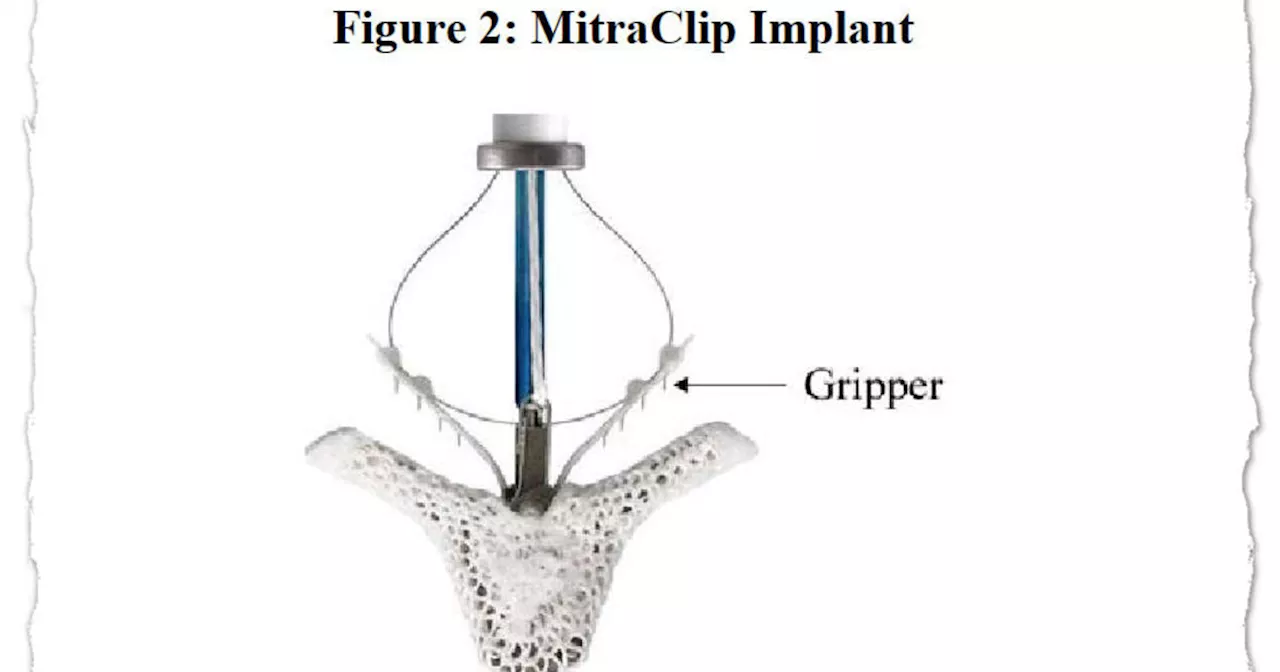
But, in May, the FDA changed its stance. The agency advised health care facilities to"transition away from these devices and seek alternatives, if possible." As a result of the FDA's May action, the company"immediately paused proactive marketing" of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.Abbott's MitraClip system includes tiny clips implanted in the heart's mitral valve and the equipment used to implant them.
Heart Heart Failure Wound Management Wound Care Injury Surgery Mitral Valve Mitral Regurgitation Mitral Valve Insufficiency Mitral Insufficiency Brain Hemorrhage Bleeding Computed Tomography CT Ct Scan Biopsy Blood Tissue Tumor Complementary And Alternative Medicine Alternative Treatment Alternative Medicine Complementary Medicine
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 The FDA calls them 'recalls,' yet the targeted medical devices often remain in useWith medical devices, recalls are not always what they seem. In some recalls, including some of the most serious, the FDA and the manufacturers let doctors and hospitals continue to use the devices.
The FDA calls them 'recalls,' yet the targeted medical devices often remain in useWith medical devices, recalls are not always what they seem. In some recalls, including some of the most serious, the FDA and the manufacturers let doctors and hospitals continue to use the devices.
Read more »
 'Discard These' Unsafe Kitchen Spices Warns FDA Amid Fresh RecallsThe FDA has expanded its cinnamon recall over high lead levels in multiple brands—check your spice rack!
'Discard These' Unsafe Kitchen Spices Warns FDA Amid Fresh RecallsThe FDA has expanded its cinnamon recall over high lead levels in multiple brands—check your spice rack!
Read more »
 FDA Issues Recalls for Chocolate Ice Cream and Candy Due to Undeclared AllergensDigital destination for sophisticated men & women. Live your best life with expert tips and news on health, food, sex, relationships, fashion and lifestyle.
FDA Issues Recalls for Chocolate Ice Cream and Candy Due to Undeclared AllergensDigital destination for sophisticated men & women. Live your best life with expert tips and news on health, food, sex, relationships, fashion and lifestyle.
Read more »
 FDA rejects MDMA for treating PTSD, calls for redo of study, drugmaker saysDrugmaker Lykos Therapeutics had asked the FDA to approve its MDMA capsules as part of a therapy regimen for treating PTSD. It says another study will take 'several years.'
FDA rejects MDMA for treating PTSD, calls for redo of study, drugmaker saysDrugmaker Lykos Therapeutics had asked the FDA to approve its MDMA capsules as part of a therapy regimen for treating PTSD. It says another study will take 'several years.'
Read more »
 FDA Calls AstraZeneca's NSCLC Trial Design Into QuestionThe agency says there's a problem with the latest trial of perioperative immunotherapy for NSCLC: There's no way to tell whether people need to continue treatment after surgery.
FDA Calls AstraZeneca's NSCLC Trial Design Into QuestionThe agency says there's a problem with the latest trial of perioperative immunotherapy for NSCLC: There's no way to tell whether people need to continue treatment after surgery.
Read more »
 Former Giant Recalls Shock Over Infamous Back-To-Back QB Sneak CallsAlex Bachman thought it was a joke when the Giants called two QB sneaks during the 2021 season.
Former Giant Recalls Shock Over Infamous Back-To-Back QB Sneak CallsAlex Bachman thought it was a joke when the Giants called two QB sneaks during the 2021 season.
Read more »
