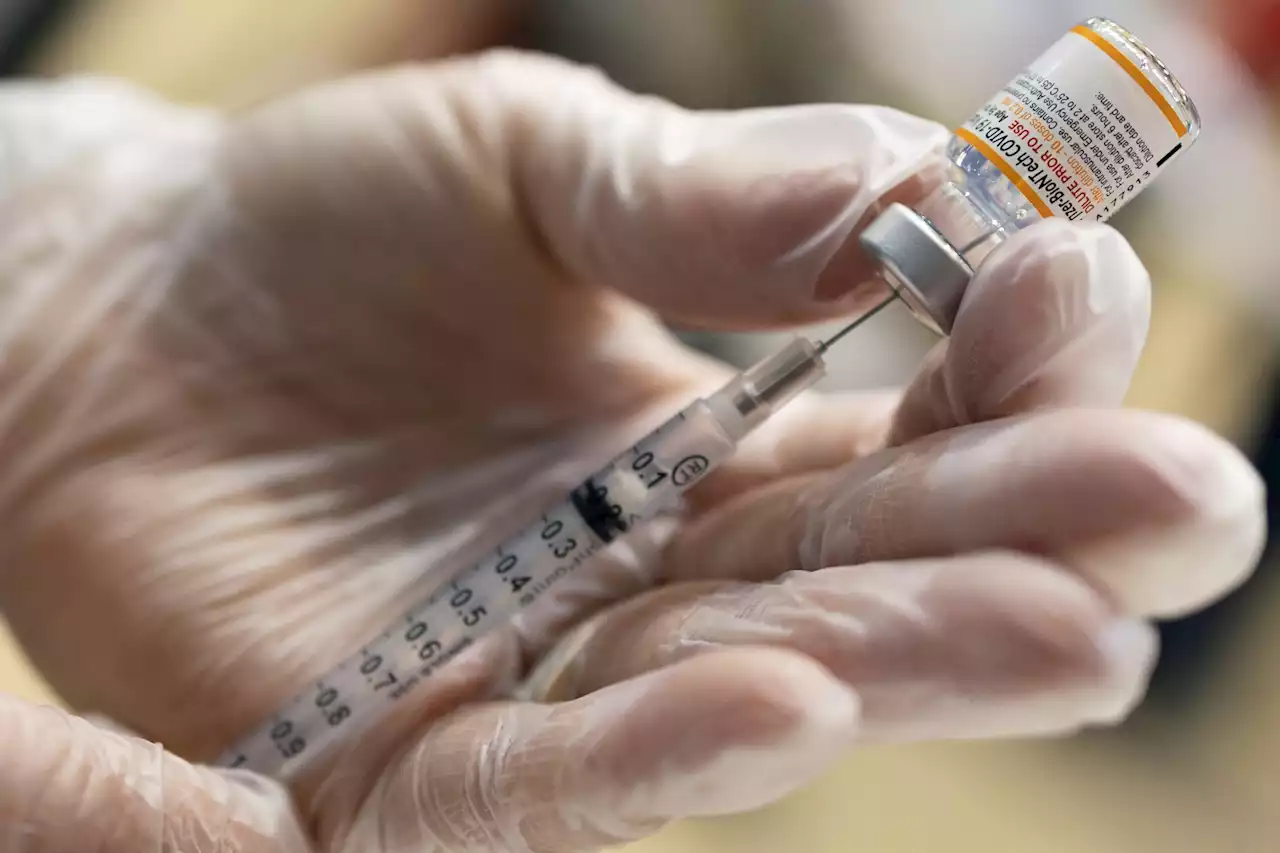
The Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
The Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series.
Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined that a third shot can help boost protection for children in this age group and the benefits outweigh the risks, Marks said.
The FDA decided to authorize a third shot after analyzing data from an ongoing Pfizer trial, in which a subset of 67 children in this age group had higher antibody levels one month after receiving a booster dose. The drug regulator did not identify any new safety concerns and found that children 5 to 11 experience the same mild side effects that other people do after receiving a booster.
FDA Commissioner Robert Califf said although Covid tends to be less severe in children, more kids have been getting sick and hospitalized with virus since the omicron variant became dominant in the U.S. over the winter.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
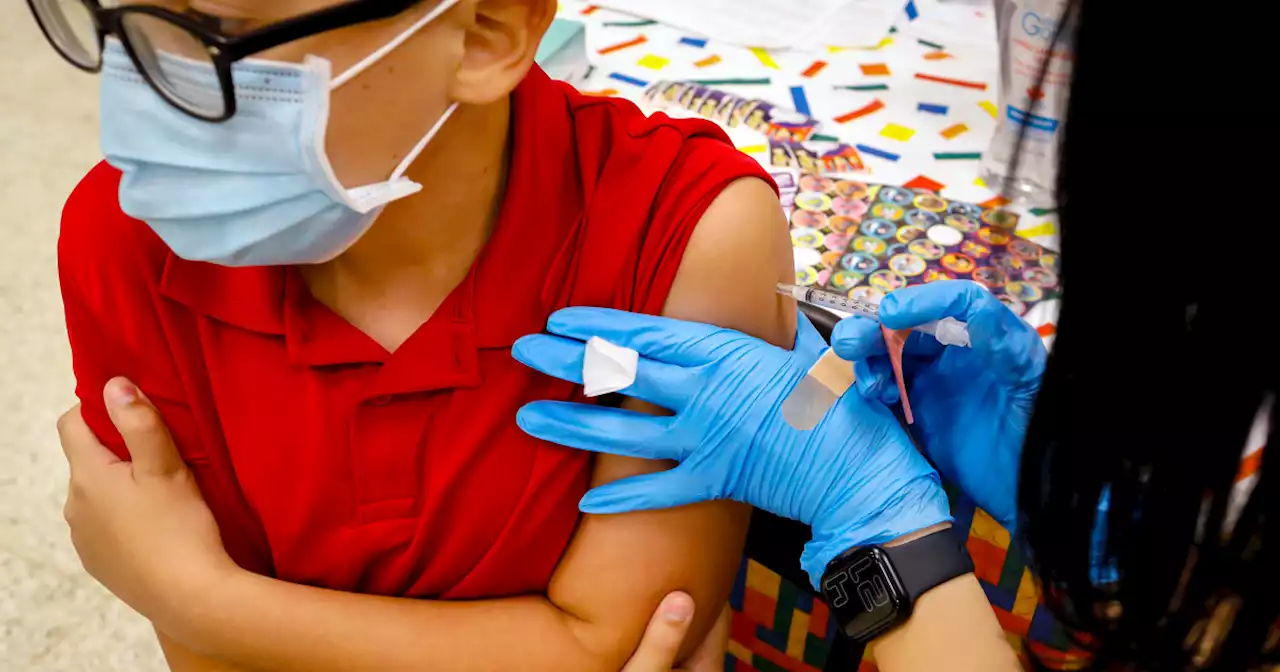 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Read more »
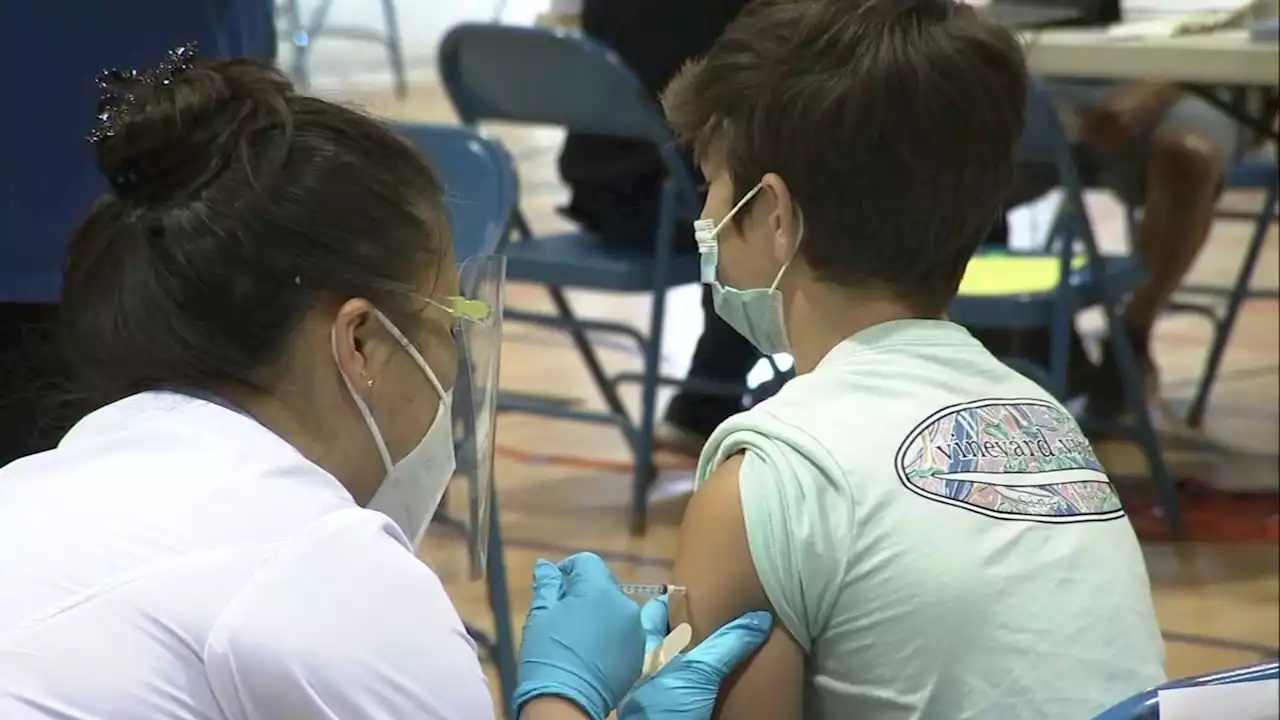 FDA gets ready to authorize vaccine booster shots for kids 5-11 weeks before summer breakAs early as Tuesday, the FDA could be authorizing Pfizer vaccine booster shots for children 5 to 11 years old -- just in time for camp and summer gatherings.
FDA gets ready to authorize vaccine booster shots for kids 5-11 weeks before summer breakAs early as Tuesday, the FDA could be authorizing Pfizer vaccine booster shots for children 5 to 11 years old -- just in time for camp and summer gatherings.
Read more »
 Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
Read more »
 FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
Read more »
 FDA could allow foreign-made infant formula to be shipped to USThe FDA could allow foreign-made infant formula to be shipped to the U.S. amid a shortage at the shelves. abc15
FDA could allow foreign-made infant formula to be shipped to USThe FDA could allow foreign-made infant formula to be shipped to the U.S. amid a shortage at the shelves. abc15
Read more »
 Major baby formula plant should re-open in about two weeks, FDA commissioner saysHe didn't say when the FDA might let the U.S. import baby formula from other countries, which could help with the massive shortage.
Major baby formula plant should re-open in about two weeks, FDA commissioner saysHe didn't say when the FDA might let the U.S. import baby formula from other countries, which could help with the massive shortage.
Read more »