The U.S. Food and Drug Administration late Friday authorized Pfizer Inc. and BioNTech SE COVID-19 bivalent vaccine for immunocompromised children. The shot,...
The U.S. Food and Drug Administration late Friday authorized Pfizer Inc. PFE and BioNTech SE BNTX COVID-19 bivalent vaccine for immunocompromised children. The shot, which would be a fourth dose in most cases, can be given to children older than 6 months through 4 years of age who have previously received the vaccine’s three doses, the FDA said. The extra dose would be administered at least 1 month following the most recent dose.
... The U.S. Food and Drug Administration late Friday authorized Pfizer Inc. PFE and BioNTech SE BNTX COVID-19 bivalent vaccine for immunocompromised children. The shot, which would be a fourth dose in most cases, can be given to children older than 6 months through 4 years of age who have previously received the vaccine’s three doses, the FDA said. The extra dose would be administered at least 1 month following the most recent dose.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
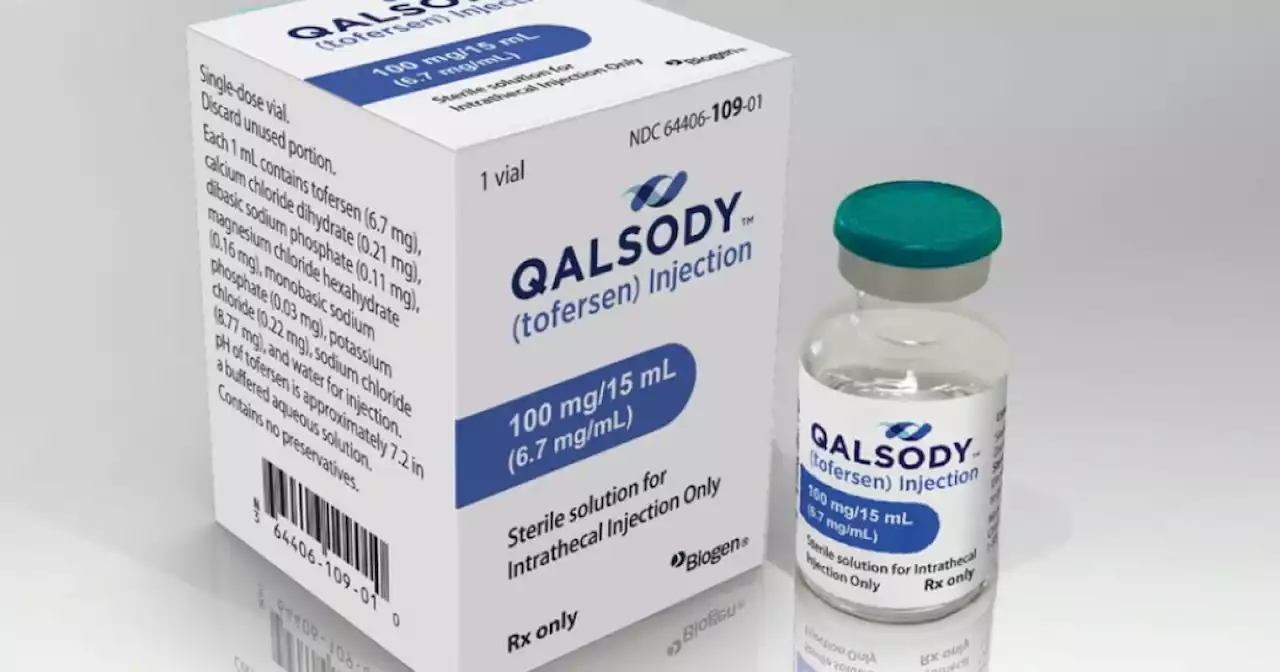 FDA approves Biogen's drug for rare form of ALSThe U.S. Food and Drug Administration has approved a new drug to treat a rare form of Amyotrophic Lateral Sclerosis (ALS), also commonly known as Lou Gehrig’s disease.
FDA approves Biogen's drug for rare form of ALSThe U.S. Food and Drug Administration has approved a new drug to treat a rare form of Amyotrophic Lateral Sclerosis (ALS), also commonly known as Lou Gehrig’s disease.
Read more »
 AstraZeneca says new COVID drug could guard against all variants of concern to dateThe drugmaker says the FDA could clear the new COVID drug for immunocompromised in months.
AstraZeneca says new COVID drug could guard against all variants of concern to dateThe drugmaker says the FDA could clear the new COVID drug for immunocompromised in months.
Read more »
 FDA Gives Fast-Track Approval to New ALS DrugThe FDA has approved the first treatment that takes a genetics-based approach to slowing or stopping the progression of a rare form of ALS, the debilitating and deadly disease for which there is no cure.
FDA Gives Fast-Track Approval to New ALS DrugThe FDA has approved the first treatment that takes a genetics-based approach to slowing or stopping the progression of a rare form of ALS, the debilitating and deadly disease for which there is no cure.
Read more »
 Pa. patient has no doubt this FDA-approved drug for ALS shows promiseOn Tuesday, the FDA granted accelerated approval to the drug, tofersen, meaning others with his type of mutation will be able to get the treatment, too — if insurers agree to pay.
Pa. patient has no doubt this FDA-approved drug for ALS shows promiseOn Tuesday, the FDA granted accelerated approval to the drug, tofersen, meaning others with his type of mutation will be able to get the treatment, too — if insurers agree to pay.
Read more »
 First pill for fecal transplants wins FDA approvalU.S. health officials on Wednesday approved the first pill made from healthy bacteria found in human waste to fight dangerous gut infections — an easier way...
First pill for fecal transplants wins FDA approvalU.S. health officials on Wednesday approved the first pill made from healthy bacteria found in human waste to fight dangerous gut infections — an easier way...
Read more »
 First pill for fecal transplants wins FDA approvalU.S. health officials have approved the first pill that uses healthy bacteria from human waste to fight dangerous gut infections — an easier way of performing so-called fecal transplants. The Food and Drug Administration approved the treatment Wednesday for adults who have trouble fighting off infections of an intestinal bug that causes thousands of deaths annually. The capsules from Seres Therapeutics are an easier-to-use version of a treatment some doctors have used for over a decade. It involves taking stool from healthy donors and using it to replenish healthy gut bacteria in patients at high risk for repeat infections.
First pill for fecal transplants wins FDA approvalU.S. health officials have approved the first pill that uses healthy bacteria from human waste to fight dangerous gut infections — an easier way of performing so-called fecal transplants. The Food and Drug Administration approved the treatment Wednesday for adults who have trouble fighting off infections of an intestinal bug that causes thousands of deaths annually. The capsules from Seres Therapeutics are an easier-to-use version of a treatment some doctors have used for over a decade. It involves taking stool from healthy donors and using it to replenish healthy gut bacteria in patients at high risk for repeat infections.
Read more »
