The U.S. Food and Drug Administration authorized several tobacco-flavored vaping products made by the company Logic, and added that it would soon announce whether other big-name brands can continue to sell their products in this country.
“We know that there is a demand among adult smokers to use e-cigarette products to try to switch from more harmful combusted cigarettes, but millions of youth are using these products and getting addicted to nicotine," FDA Commissioner Dr. Robert Califf noted in an agency."The balance of these issues was considered by the agency’s career scientists when evaluating the potential marketing of e-cigarette products.
While the FDA approved several tobacco-flavored products from Logic, it said no to some of the company's other products and has yet to make a decision on others, including some with menthol. "This is yet another baby step when, unfortunately, our babies which have become young adults are getting addicted every day to more and more of these products," Matthew Myers, president of the Campaign for Tobacco-Free Kids, told"Today's decision authorizing a tobacco-flavored e-cigarette that is not widely used by either adults or youth doesn't set any new precedent," Myers said."The most important decisions are still sitting in front of the FDA.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
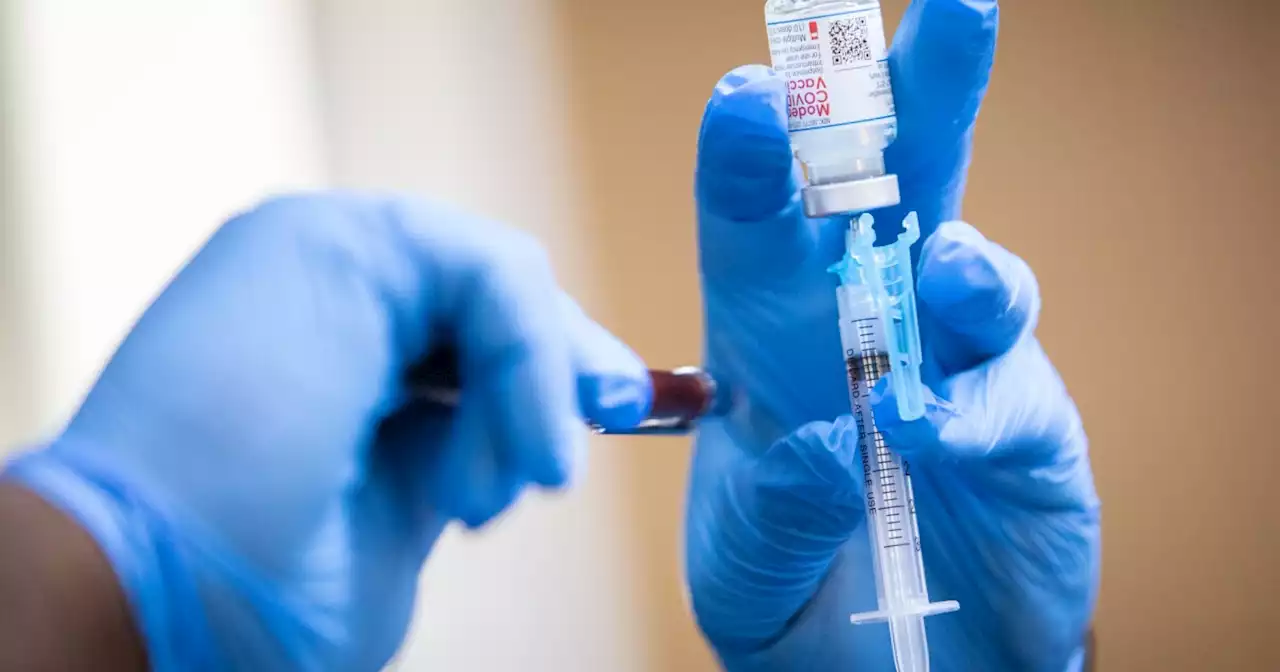 Moderna wants the FDA to authorize its COVID-19 vaccine for kids under 6Moderna wants the FDA to authorize its COVID-19 vaccine for kids under six. Here's what's next in the process. | via NPR
Moderna wants the FDA to authorize its COVID-19 vaccine for kids under 6Moderna wants the FDA to authorize its COVID-19 vaccine for kids under six. Here's what's next in the process. | via NPR
Read more »
 U.S. FDA approves Novartis therapy for prostate cancerThe U.S. Food and Drug Administration approved Novartis AG's therapy for the treatment of patients with a type of advanced prostate cancer that has spread to other parts of the body, the drugmaker said on Wednesday.
U.S. FDA approves Novartis therapy for prostate cancerThe U.S. Food and Drug Administration approved Novartis AG's therapy for the treatment of patients with a type of advanced prostate cancer that has spread to other parts of the body, the drugmaker said on Wednesday.
Read more »
 Google asks FDA to clear Fitbit for passive heart rhythm monitoringA new feature would run in the background
Google asks FDA to clear Fitbit for passive heart rhythm monitoringA new feature would run in the background
Read more »
 FDA Rejects Eli Lilly’s China-Developed Cancer DrugThe agency asked Lilly and its China-based partner to conduct another trial of the immunotherapy in multiple regions.
FDA Rejects Eli Lilly’s China-Developed Cancer DrugThe agency asked Lilly and its China-based partner to conduct another trial of the immunotherapy in multiple regions.
Read more »
 Moderna will seek FDA emergency use authorization for its vaccine in kids under 6
Moderna will seek FDA emergency use authorization for its vaccine in kids under 6
Read more »
 Eli Lilly's BLA for sintilimab can't be approved by FDA in present formEli Lilly & Co. undefined said Thursday it received a complete response letter (CRL) from the U.S. Food and Drug Administration regarding its Biologics...
Eli Lilly's BLA for sintilimab can't be approved by FDA in present formEli Lilly & Co. undefined said Thursday it received a complete response letter (CRL) from the U.S. Food and Drug Administration regarding its Biologics...
Read more »
