JUST IN | The FDA amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
Pfizer says the booster shot provided a"strong immune response" in children who took part in a clinical trial.“The Pfizer-BioNTech COVID-19 Vaccine is effective in helping to prevent the most severe consequences of COVID-19 in individuals 5 years of age and older,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
FDA Commissioner Robert M. Califf acknowledged that COVID-19 tends to be less severe in children than adults. He noted, however, that more children have gotten sick and been treated in the hospital during the omicron wave. "Children may also experience longer term effects, even following initially mild disease," Califf said.
The FDA says the best protection against COVID-19 is getting vaccinated. However, there is still no vaccine available for children under five.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Read more »
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
Read more »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
Read more »
 FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
Read more »
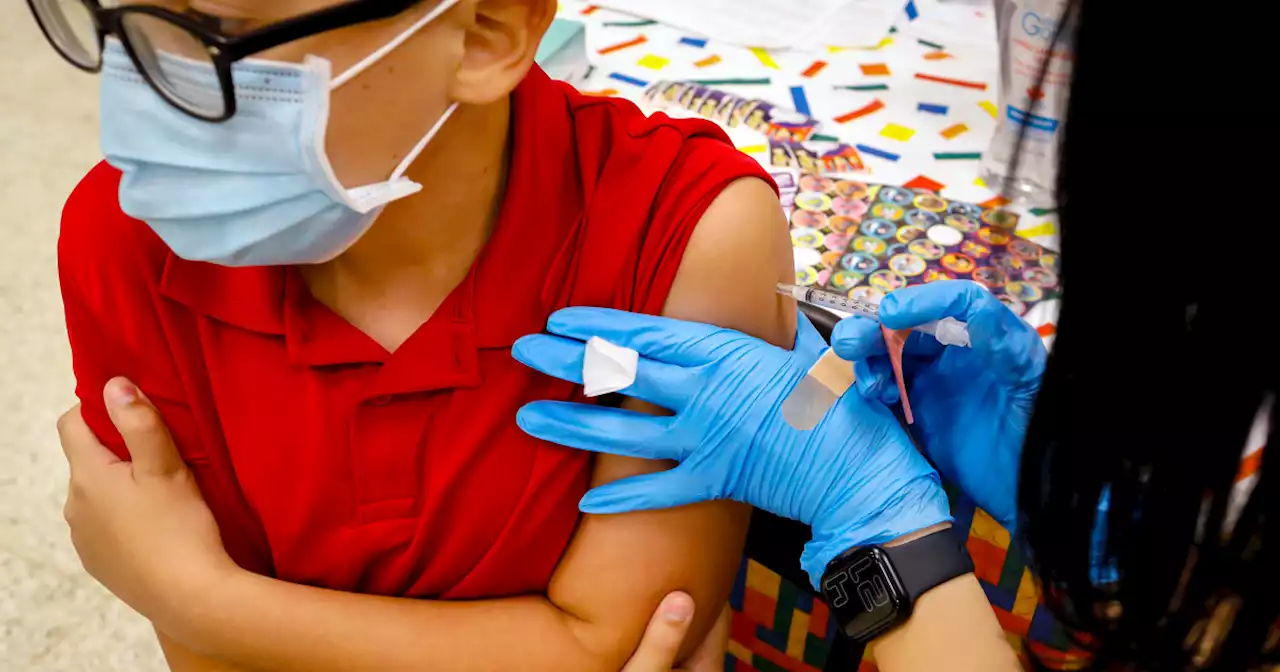 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Read more »
