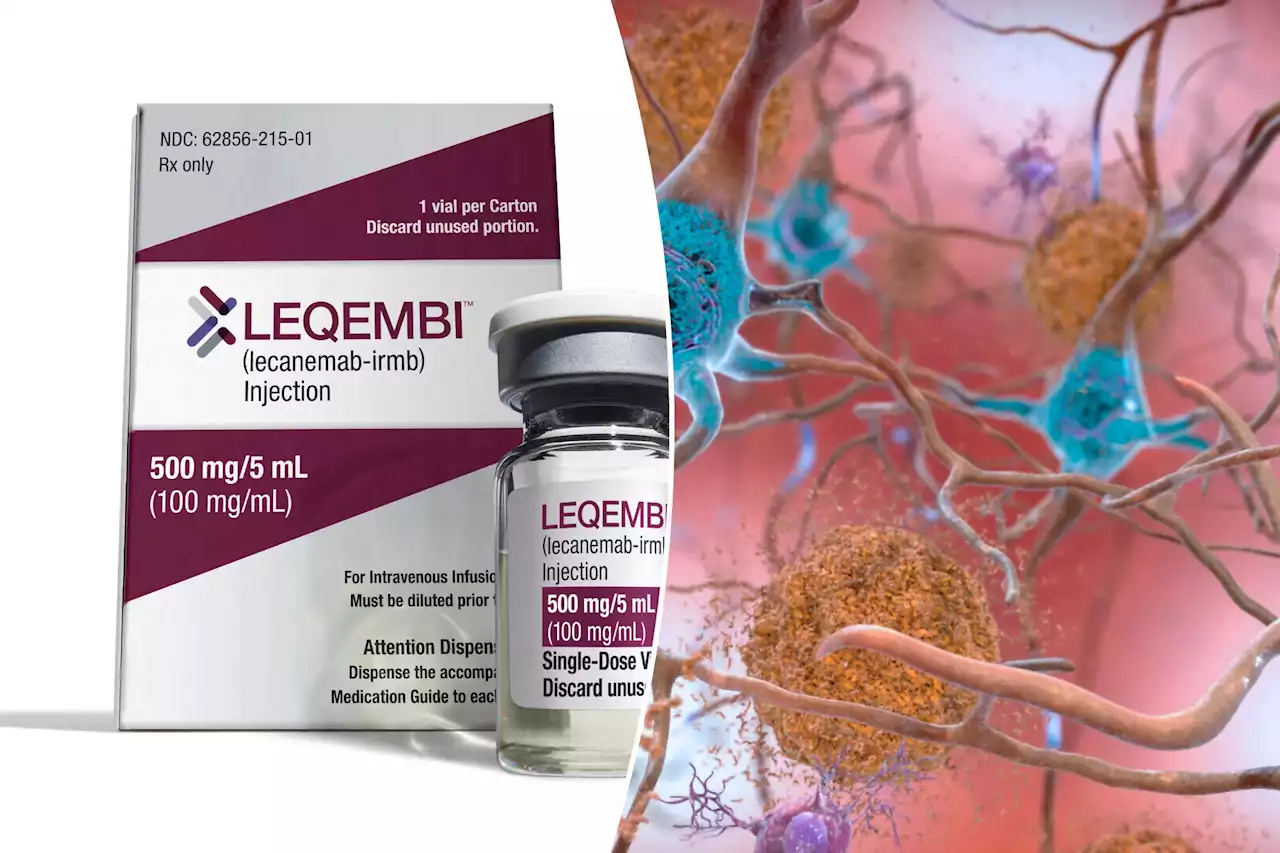
Lecanemab, marketed as Leqembi, could be a game-changer for people with Alzheimer’s disease — but only if they can get it.
A Food and Drug Administration panel has unanimously approved the use of lecanemab to slow the progression of Alzheimer’s disease.
But even though the FDA approved lecanemab through an accelerated approval process, the Centers for Medicare and Medicaid Services — which controls Medicare — will only cover the drug for patients who enroll in a national registry. That roadblock “is an unnecessary and potentially harmful barrier,” according to the Alzheimer’s Association.“Medicare is supposed to be a rock-solid guarantee for Americans, and it is time for CMS to step up and provide Medicare access on the day of an FDA traditional approval,”“Americans living with Alzheimer’s disease deserve access to FDA-approved therapies without barriers, just like people with cancer, heart disease and HIV/AIDS,” the advocacy group added.
Providing coverage for new and effective drugs for Alzheimer’s and other forms of dementia would save the public payers between $13.1 billion and $545.6 billion in health care costs over the course of 17 years,The CMS wants more information about lecanemab, even though the Department of Veterans Affairs has already decided to use it for patients in the VA system.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Panel Unanimously Endorses Lecanemab for Alzheimer'sFDA advisory committee determines phase 3 study of lecanemab, a monoclonal antibody directed against amyloid beta confirms the drug's clinical benefit for patients with Alzheimer's disease.
FDA Panel Unanimously Endorses Lecanemab for Alzheimer'sFDA advisory committee determines phase 3 study of lecanemab, a monoclonal antibody directed against amyloid beta confirms the drug's clinical benefit for patients with Alzheimer's disease.
Read more »
 FDA paves way for full approval of new Alzheimer's drugA panel of Food and Drug Administration advisers on Friday unanimously endorsed an experimental Alzheimer's drug that's been shown to have modest success slowing the progression of the disease, clearing a hurdle for full agency approval.
FDA paves way for full approval of new Alzheimer's drugA panel of Food and Drug Administration advisers on Friday unanimously endorsed an experimental Alzheimer's drug that's been shown to have modest success slowing the progression of the disease, clearing a hurdle for full agency approval.
Read more »
 FDA Flags Getinge/Maquet Recall of Oxygenator DevicesGetinge/Maquet recalls all Quadrox oxygenators and certain venous hardshell cardiotomy reservoirs due to a potential sterility issue with packaging that could lead to risk for infection and patient harm.
FDA Flags Getinge/Maquet Recall of Oxygenator DevicesGetinge/Maquet recalls all Quadrox oxygenators and certain venous hardshell cardiotomy reservoirs due to a potential sterility issue with packaging that could lead to risk for infection and patient harm.
Read more »
 Alzheimer's drug gets FDA panel's backing, setting the stage for broader useHealth advisers on Friday unanimously backed the full approval of a closely watched Alzheimer's drug, a key step toward opening insurance coverage to U.S. seniors with early stages of the brain-robbing disease.
Alzheimer's drug gets FDA panel's backing, setting the stage for broader useHealth advisers on Friday unanimously backed the full approval of a closely watched Alzheimer's drug, a key step toward opening insurance coverage to U.S. seniors with early stages of the brain-robbing disease.
Read more »
 Updated Covid vaccines need to target XBB omicron variants this fall, FDA staff saysVaccine manufacturers Pfizer, Moderna and Novavax will be expected to update their shots according to the strain the FDA selects for the fall.
Updated Covid vaccines need to target XBB omicron variants this fall, FDA staff saysVaccine manufacturers Pfizer, Moderna and Novavax will be expected to update their shots according to the strain the FDA selects for the fall.
Read more »
 Opinion: New Alzheimer's drugs are costly and controversial. Are we going about this all wrong?New medications such as lecanemab offer at best modest effects and major expenses. But dementia can be forestalled with practical and affordable measures.
Opinion: New Alzheimer's drugs are costly and controversial. Are we going about this all wrong?New medications such as lecanemab offer at best modest effects and major expenses. But dementia can be forestalled with practical and affordable measures.
Read more »