The U.S. Food and Drug Administration is approving more novel pharmaceutical drugs based on single clinical trials and with less public disclosure about those trials than was the norm just a few years ago, a pair of recent studies from Oregon State University found.
Researchers agree it is important to minimize delays in making treatments for diseases such as cancer available to patients, but they say their findings point to a need for greater transparency around how drugs receive approval.
As part of that law, the FDA relaxed some standards to allow treatments for priority health conditions such as cancer to be approved with fewer supporting studies, and placed less emphasis on randomized clinical trials, allowing pharmaceutical companies to rely on surrogate markers instead of clinical outcomes in certain cases. Surrogate markers are used as substitutes when the direct clinical outcomes take a long time to study, and they should be related to the clinical outcomes.
Related StoriesResearchers also looked at the availability of drug trial results on the public-facing ClinicalTrials.gov, a database maintained by the National Institutes of Health that patients can use to learn more about drugs they may be prescribed. That doesn't necessarily mean the FDA is denied access to those full results, Irvin said, but the public cannot read the results until they are posted publicly.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 New waterfront food and drink village reveals full lineup of street food tradersThe food and drink destination will feature a food hall and street kitchens making it the largest of its kind in Greater Manchester
New waterfront food and drink village reveals full lineup of street food tradersThe food and drink destination will feature a food hall and street kitchens making it the largest of its kind in Greater Manchester
Read more »
 FDA approving drugs after fewer trials, providing less information to public, studies findThe U.S. Food and Drug Administration is approving more novel pharmaceutical drugs based on single clinical trials and with less public disclosure about those trials than was the norm just a few years ago, a pair of recent studies from Oregon State University has found.
FDA approving drugs after fewer trials, providing less information to public, studies findThe U.S. Food and Drug Administration is approving more novel pharmaceutical drugs based on single clinical trials and with less public disclosure about those trials than was the norm just a few years ago, a pair of recent studies from Oregon State University has found.
Read more »
 Don't use Dr. Berne's and LightEyez eye drops due to bacteria, fungus, FDA saysTainted eye drops are back in the news, with federal regulators warning consumers not to use certain eye drops because of contamination concerns.
Don't use Dr. Berne's and LightEyez eye drops due to bacteria, fungus, FDA saysTainted eye drops are back in the news, with federal regulators warning consumers not to use certain eye drops because of contamination concerns.
Read more »
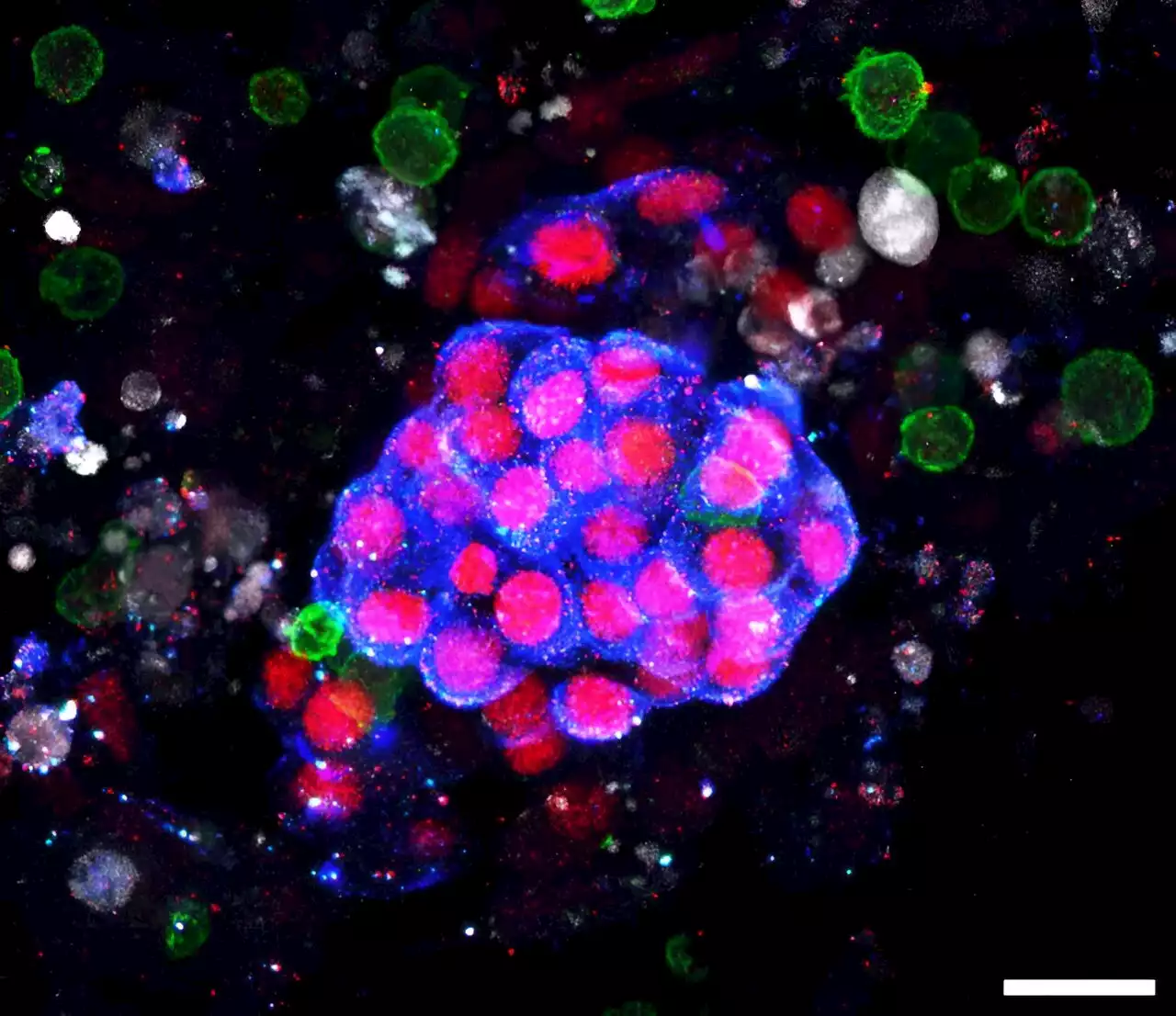 Adding immunity to human kidney-on-a-chip advances cancer drug testingA growing repertoire of cell and molecule-based immunotherapies is offering patients with indomitable cancers new hope by mobilizing their immune systems against tumor cells. An emerging class of such immunotherapeutics, known as T cell bispecific antibodies (TCBs), are of growing importance with several TCBs that the U.S. Food and Drug Administration (FDA) approved for the treatment of leukemias, lymphomas, and myelomas.
Adding immunity to human kidney-on-a-chip advances cancer drug testingA growing repertoire of cell and molecule-based immunotherapies is offering patients with indomitable cancers new hope by mobilizing their immune systems against tumor cells. An emerging class of such immunotherapeutics, known as T cell bispecific antibodies (TCBs), are of growing importance with several TCBs that the U.S. Food and Drug Administration (FDA) approved for the treatment of leukemias, lymphomas, and myelomas.
Read more »
 Downfall of £2m drug gang who used Sports Direct bags to move cannabisWaqas Mahmood, Xavier Khalid, Asif Iqbal, Mihai Dumitru and Philip Wilkinson used Vietnamese nationals to cultivate and harvest the cannabis at factories in Burnley and Pendle
Downfall of £2m drug gang who used Sports Direct bags to move cannabisWaqas Mahmood, Xavier Khalid, Asif Iqbal, Mihai Dumitru and Philip Wilkinson used Vietnamese nationals to cultivate and harvest the cannabis at factories in Burnley and Pendle
Read more »
 Glasgow gangland enforcers targeted frightened parents over sons £80k drug debtRobert Notman tried to extort money from the addict's mother by targeting her home and demanding she repay her son's £80,000 debt.
Glasgow gangland enforcers targeted frightened parents over sons £80k drug debtRobert Notman tried to extort money from the addict's mother by targeting her home and demanding she repay her son's £80,000 debt.
Read more »
