The U.S. Food and Drug Administration has approved Kisqali for early-stage breast cancer, citing a 25% reduction in recurrence risk based on a phase III trial. This approval provides women diagnosed with early-stage breast cancer with an additional option to help prevent the cancer from returning.
The U.S. Food and Drug Administration has given approval to the metastatic breast cancer Kisqali , which was found to reduce the risk of cancer recurrence by 25%. The approval will give women diagnosed with early-stage breast cancer access to a drug to try and help prevent cancer cases from coming back. A phase III trial showed 'a significant and clinically meaningful 25.1% reduction in risk,' Novartis said in a statement.
Hospitals begin offering breakthrough radiation therapy for metastatic cancer tumorsDr. Dennis J. Slamon, a lead investigator in the trial, said 'The FDA approval of Kisqali for this early breast cancer population ... is a pivotal moment in improving our approach to care.'Novartis said around 90% of breast cancer cases in the U.S. are diagnosed in their early stages.
Breast Cancer Kisqali FDA Approval Recurrence Risk Early-Stage Cancer
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA expands approval of breast cancer drug Kisqali to earlier stage patientsThe FDA expanded the approval of Kisqali, a drug for metastatic breast cancer, to also treat patients with earlier stages of the disease.
FDA expands approval of breast cancer drug Kisqali to earlier stage patientsThe FDA expanded the approval of Kisqali, a drug for metastatic breast cancer, to also treat patients with earlier stages of the disease.
Read more »
 FDA Expands Use of Breast Cancer Drug KisqaliWomen with early stage breast cancer may now take Kisquali, a medication already approved for advanced disease, following the U.S. Food and Drug Administration's expanded approval of the treatment, drug maker Novartis announced Tuesday.
FDA Expands Use of Breast Cancer Drug KisqaliWomen with early stage breast cancer may now take Kisquali, a medication already approved for advanced disease, following the U.S. Food and Drug Administration's expanded approval of the treatment, drug maker Novartis announced Tuesday.
Read more »
 Breast cancer drug Kisqali gets FDA approval to prevent cancer recurrenceRooted in fact-based, transparent reporting, Newsy is an award-winning opinion-free network owned by the E.W. Scripps Company that is relentlessly focused on “the why” of every story and seeks to enable a more intimate and immersive understanding of the issues that matter.
Breast cancer drug Kisqali gets FDA approval to prevent cancer recurrenceRooted in fact-based, transparent reporting, Newsy is an award-winning opinion-free network owned by the E.W. Scripps Company that is relentlessly focused on “the why” of every story and seeks to enable a more intimate and immersive understanding of the issues that matter.
Read more »
 FDA Approves Benralizumab for EGPA Vasculitis IndicationIn a clinical trial, nearly 60% of participants achieved remission and 41% fully tapered off oral corticosteroids.
FDA Approves Benralizumab for EGPA Vasculitis IndicationIn a clinical trial, nearly 60% of participants achieved remission and 41% fully tapered off oral corticosteroids.
Read more »
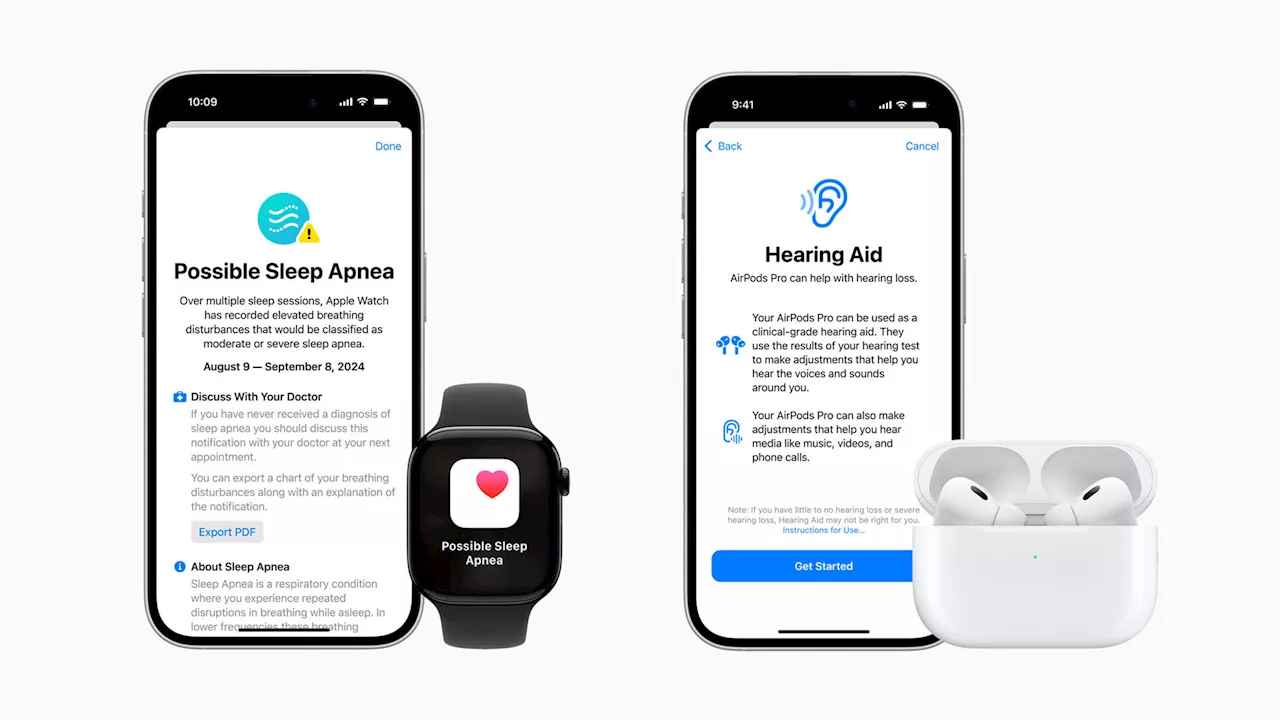 FDA approves Apple Watch screening for sleep apnea riskMack DeGeurin is a tech reporter who’s spent years investigating where technology and politics collide. His work has previously appeared in Gizmodo, Insider, New York Magazine, and Vice.
FDA approves Apple Watch screening for sleep apnea riskMack DeGeurin is a tech reporter who’s spent years investigating where technology and politics collide. His work has previously appeared in Gizmodo, Insider, New York Magazine, and Vice.
Read more »
 FDA Approves Sleep Apnea Detection on Apple WatchAleksandar is a tech enthusiast with a broad range of interests, from smartphones to space exploration. His curiosity extends to hands-on DIY experiments with his gadgets, and he enjoys switching between different brands to experience the latest innovations.
FDA Approves Sleep Apnea Detection on Apple WatchAleksandar is a tech enthusiast with a broad range of interests, from smartphones to space exploration. His curiosity extends to hands-on DIY experiments with his gadgets, and he enjoys switching between different brands to experience the latest innovations.
Read more »
