After a delayed approval, treosulfan’s manufacturer is hopeful the alkylating agent will become the new gold standard in the United States.
, a Medexus executive said the company is “optimistic” that treosulfan will become the new “gold standard” in the United States for the indication “as it in Europe and Canada,” where the alkylating agent is already on the market.
Approval in the United States was delayed for several years pending FDA’s request for additional information regarding treosulfan’s pivotal phase 3 trial, dubbed The trial included European adults with acute myeloid leukemia or myelodysplastic syndromes undergoing transplant. They were randomly assigned about equally to intravenous fludarabine with either treosulfan at 30 g/mat 6.4 mg/kg IV, which is a reduced-intensity dose of busulfan widely used in older patients or in those with comorbidity., fatigue, infections, and febrile neutropenia.
The phase 3 trial reported equal distribution of such adverse events between the treosulfan and busulfan study arms. M. Alexander Otto is a physician assistant with a master’s degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape. Alex is also an MIT Knight Science Journalism fellow. Email:FDA Review Skeptical of ALS Stem Cell Therapy Ahead of Panel MeetingAll material on this website is protected by copyright, Copyright © 1994-2025 by WebMD LLC.
Hematopoietic Stem Cell Transplant Stem Cell Transplantation Stem Cell Therapy Haematopoietic Stem Cell Transplant Hematopoietic Stem Cell Transplantation (HSCT) Haematopoietic Stem Cell Transplantation (HSCT) Stem Cell Research And Therapy Transplantation Of Stem Cells Transplantation Of Hematopoietic Stem Cells Stem Cell HSCT Acute Leukemia
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves first medication for obstructive sleep apnea, which also promotes weight lossThe FDA approved a new drug treatment for obstructive sleep apnea that improves the condition through weight loss. Sleep expert Dr. Wendy Troxel discusses the impact of the new development.
FDA approves first medication for obstructive sleep apnea, which also promotes weight lossThe FDA approved a new drug treatment for obstructive sleep apnea that improves the condition through weight loss. Sleep expert Dr. Wendy Troxel discusses the impact of the new development.
Read more »
 FDA Approves Generic GLP-1 Medicine For Diabetes TreatmentThe U.S. Food and Drug Administration (FDA) announced on Monday the approval of the first generic version of a daily injectable GLP-1 medicine for people living with Type 2 diabetes.
FDA Approves Generic GLP-1 Medicine For Diabetes TreatmentThe U.S. Food and Drug Administration (FDA) announced on Monday the approval of the first generic version of a daily injectable GLP-1 medicine for people living with Type 2 diabetes.
Read more »
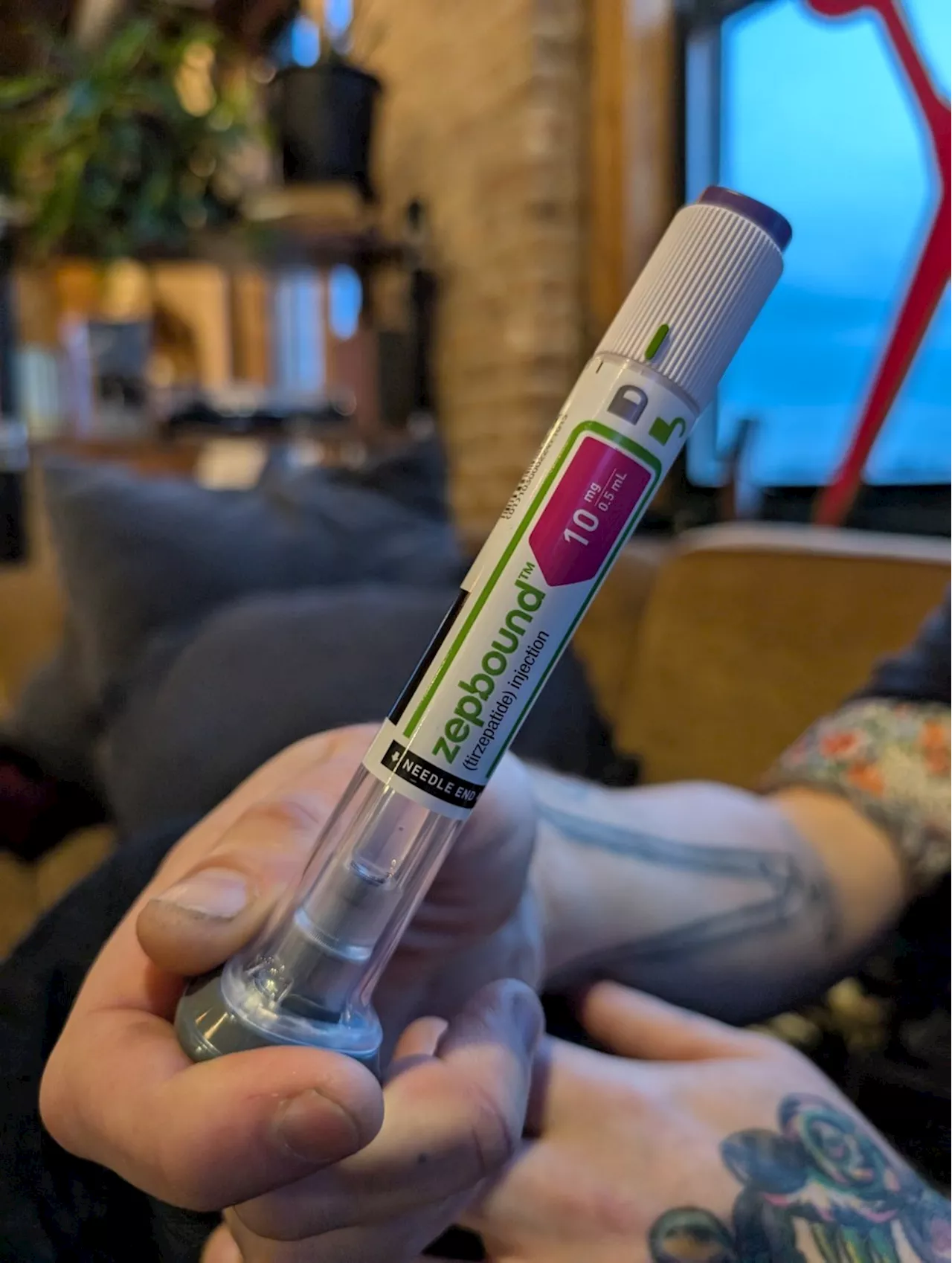 FDA approves Eli Lilly's Zepbound for sleep apnea treatmentThe FDA granted approval to Eli Lilly's weight-loss drug Zepbound for treating sleep apnea, marking a significant advancement in managing this prevalent condition.
FDA approves Eli Lilly's Zepbound for sleep apnea treatmentThe FDA granted approval to Eli Lilly's weight-loss drug Zepbound for treating sleep apnea, marking a significant advancement in managing this prevalent condition.
Read more »
 FDA Approves 50 New Drugs in 2024, Marking Firsts for Several ConditionsThe U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, ushering in a wave of groundbreaking treatments for various diseases. Among the notable approvals were the first-ever medications for metabolic dysfunction-associated steatohepatitis (MASH) and Niemann-Pick disease type C.
FDA Approves 50 New Drugs in 2024, Marking Firsts for Several ConditionsThe U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, ushering in a wave of groundbreaking treatments for various diseases. Among the notable approvals were the first-ever medications for metabolic dysfunction-associated steatohepatitis (MASH) and Niemann-Pick disease type C.
Read more »
 FDA Approves Record Number of New Drugs in 2024The U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, marking a significant year for medical advancements. The approvals included groundbreaking treatments for previously incurable diseases, such as metabolic dysfunction-associated steatohepatitis (MASH) and Niemann-Pick disease type C.
FDA Approves Record Number of New Drugs in 2024The U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, marking a significant year for medical advancements. The approvals included groundbreaking treatments for previously incurable diseases, such as metabolic dysfunction-associated steatohepatitis (MASH) and Niemann-Pick disease type C.
Read more »
 FDA Approves Wave of New Treatments in 2024The U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, including groundbreaking treatments for various diseases.
FDA Approves Wave of New Treatments in 2024The U.S. Food and Drug Administration (FDA) concluded 2024 with 50 new drug approvals, including groundbreaking treatments for various diseases.
Read more »