Spravato (esketamine) nasal spray has received FDA approval for adults with major depressive disorder who haven't responded to traditional treatments. The drug, administered under healthcare supervision, targets the neurotransmitter glutamate and shows promise in reducing depressive symptoms within 24 hours. A restricted access program will ensure safe and appropriate use due to potential sedation, dissociation, and respiratory risks.
The U.S. Food and Drug Administration has approved Spravato CIII nasal spray for adults living with major depressive disorder who have had an inadequate response to at least two oral Spravato is the first and only approved monotherapy for adults with refractory major depressive disorder. Approval of Spravato , granted following FDA priority review, was based on the results of a randomized, double-blind, multicenter, placebo-controlled trial.
Spravato nasal spray is administered by the patient under the supervision of a health care provider in a health care setting. Spravato targets the neurotransmitter glutamate; however, the mechanism by which esketamine exerts itseffect is unknown. In an effort to ensure the safe and appropriate use of Spravato, the medication is only available through a restricted program called the Spravato Risk Evaluation and Mitigation Strategy Program.
Depression Treatment FDA Approval Spravato Esketamine
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Approves First Nasal Spray for Major Depressive DisorderThe FDA has approved SPRAVATO, a nasal spray that offers a new treatment option for individuals with major depressive disorder who haven't responded well to oral antidepressants.
FDA Approves First Nasal Spray for Major Depressive DisorderThe FDA has approved SPRAVATO, a nasal spray that offers a new treatment option for individuals with major depressive disorder who haven't responded well to oral antidepressants.
Read more »
 FDA approves first-of-its-kind nasal spray to treat depressionJohnson & Johnson's ketamine-derived nasal spray has been approved as a standalone treatment to fight depression. Here's what to know.
FDA approves first-of-its-kind nasal spray to treat depressionJohnson & Johnson's ketamine-derived nasal spray has been approved as a standalone treatment to fight depression. Here's what to know.
Read more »
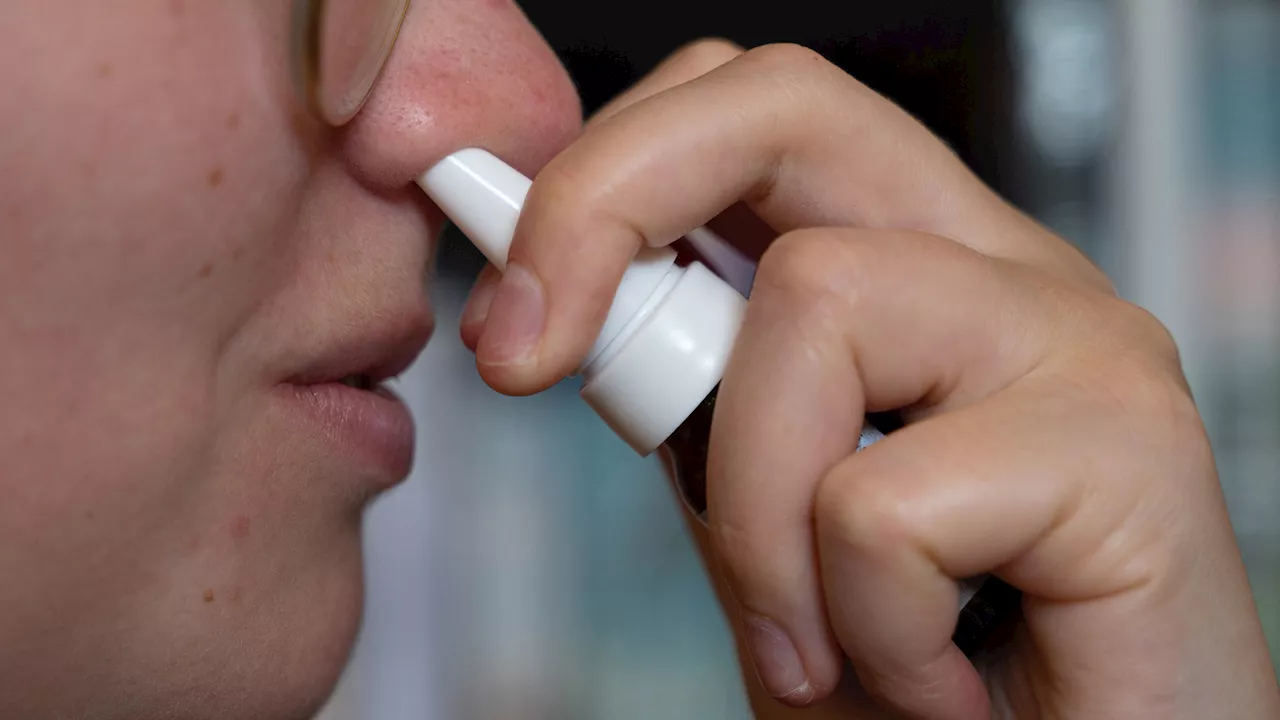 FDA Approves First-of-Its-Kind Nasal Spray for Treatment-Resistant DepressionThe U.S. Food and Drug Administration (FDA) has approved Spravato, a nasal spray containing esketamine, as a standalone treatment for major depressive disorder (MDD), post-traumatic stress disorder (PTSD), and other similar psychiatric conditions. This marks a significant advancement in the treatment of treatment-resistant depression, offering hope to millions of Americans who haven't found relief with traditional antidepressants.
FDA Approves First-of-Its-Kind Nasal Spray for Treatment-Resistant DepressionThe U.S. Food and Drug Administration (FDA) has approved Spravato, a nasal spray containing esketamine, as a standalone treatment for major depressive disorder (MDD), post-traumatic stress disorder (PTSD), and other similar psychiatric conditions. This marks a significant advancement in the treatment of treatment-resistant depression, offering hope to millions of Americans who haven't found relief with traditional antidepressants.
Read more »
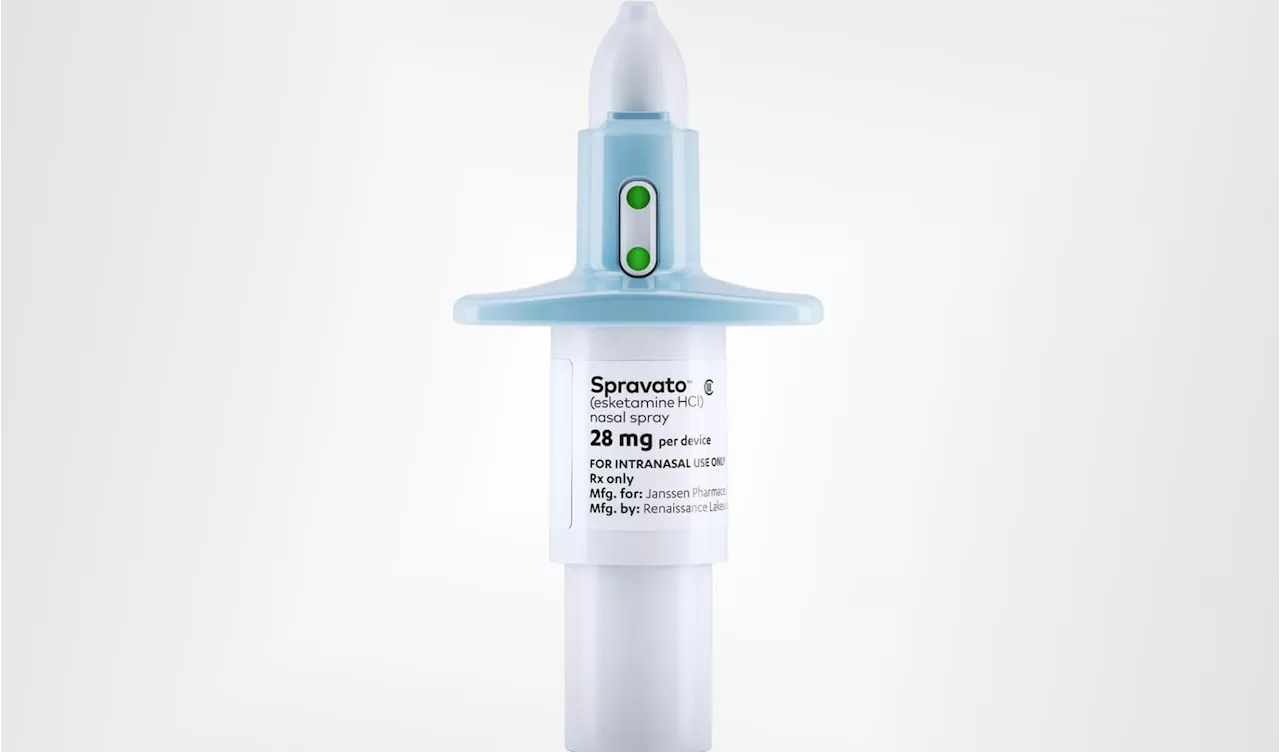 FDA approves Johnson & Johnson's nasal spray for depression as standalone treatmentSpravato is now the first-ever standalone therapy for treatment-resistant depression, and is on its way to becoming a blockbuster product.
FDA approves Johnson & Johnson's nasal spray for depression as standalone treatmentSpravato is now the first-ever standalone therapy for treatment-resistant depression, and is on its way to becoming a blockbuster product.
Read more »
 FDA Approves Johnson & Johnson's Ketamine Nasal Spray for Severe DepressionThe Food and Drug Administration (FDA) has approved Spravato, a ketamine-derived nasal spray developed by Johnson & Johnson, as a groundbreaking treatment for severe depression. This marks the first standalone therapy specifically for adults with major depressive disorder who haven't responded to traditional antidepressants.
FDA Approves Johnson & Johnson's Ketamine Nasal Spray for Severe DepressionThe Food and Drug Administration (FDA) has approved Spravato, a ketamine-derived nasal spray developed by Johnson & Johnson, as a groundbreaking treatment for severe depression. This marks the first standalone therapy specifically for adults with major depressive disorder who haven't responded to traditional antidepressants.
Read more »
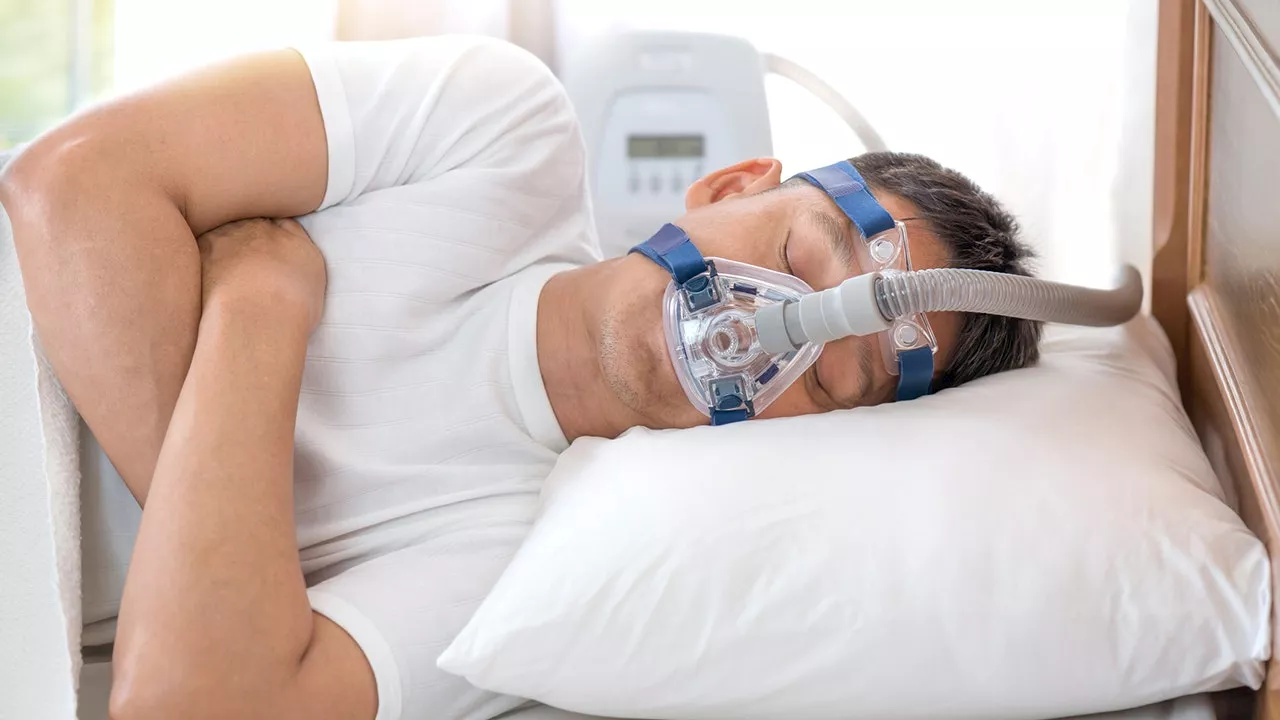 FDA approves first medication for obstructive sleep apnea, which also promotes weight lossThe FDA approved a new drug treatment for obstructive sleep apnea that improves the condition through weight loss. Sleep expert Dr. Wendy Troxel discusses the impact of the new development.
FDA approves first medication for obstructive sleep apnea, which also promotes weight lossThe FDA approved a new drug treatment for obstructive sleep apnea that improves the condition through weight loss. Sleep expert Dr. Wendy Troxel discusses the impact of the new development.
Read more »
