The FDA has approved Zepbound (tirzepatide) for adults with obesity, marking a major advancement in treating obstructive sleep apnea.
The U.S. Food and Drug Administration (FDA) has approved Zepbound (tirzepatide) by Eli Lilly to treat moderate to severe obstructive sleep apnea (OSA) in adults with obesity. The drug is to be used alongside a reduced-calorie diet and increased physical activity. The FDA noted that this is the first medication approved to treat OSA. OSA occurs when the upper airway becomes blocked, causing breathing pauses during sleep, and is more common in overweight or obese individuals.
Zepbound works similar to semaglutide treatments like Ozempic and Wegovy by activating receptors of hormones secreted from the intestine (GLP-1 and GIP) to reduce appetite and food intake. Studies have shown that by reducing body weight, Zepbound also improves OSA. In a 52-week study, participants treated with Zepbound experienced a statistically significant and clinically meaningful reduction in apnea or hypopnea events, with a large share achieving remission or resolution of symptoms. Zepbound-treated patients also reported a significant decrease in body weight
Obstructive Sleep Apnea FDA Approval Medication Obesity Tirzepatide
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
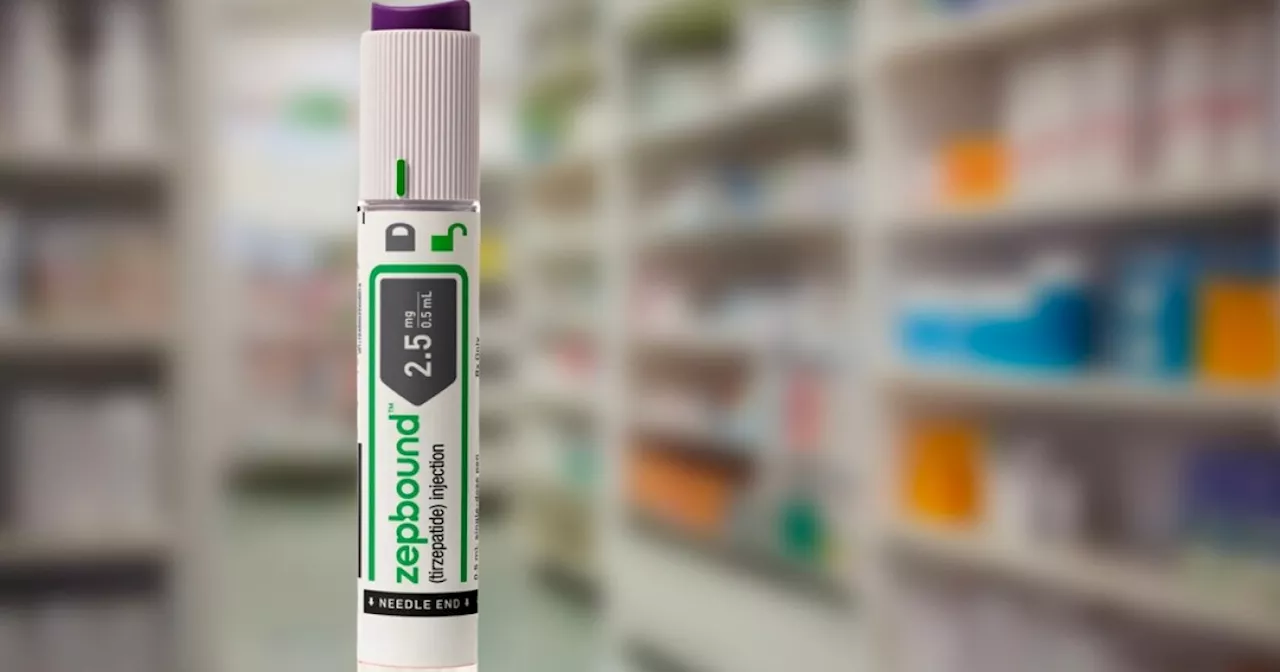 FDA approves first-ever medication for obstructive sleep apneaJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
FDA approves first-ever medication for obstructive sleep apneaJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
Read more »
 FDA Approves First Allogeneic MSC Therapy for Steroid-Refractory Acute Graft-vs-Host Disease in ChildrenRyoncil, a mesenchymal stromal cell therapy derived from bone marrow, has received FDA approval for the treatment of steroid-refractory acute graft-vs-host disease (SR-aGVHD) in children aged 2 months or older. The approval marks a significant advancement in cell-based therapies for life-threatening diseases. In a phase 3 study, Ryoncil demonstrated effectiveness in inducing responses in children with SR-aGVHD.
FDA Approves First Allogeneic MSC Therapy for Steroid-Refractory Acute Graft-vs-Host Disease in ChildrenRyoncil, a mesenchymal stromal cell therapy derived from bone marrow, has received FDA approval for the treatment of steroid-refractory acute graft-vs-host disease (SR-aGVHD) in children aged 2 months or older. The approval marks a significant advancement in cell-based therapies for life-threatening diseases. In a phase 3 study, Ryoncil demonstrated effectiveness in inducing responses in children with SR-aGVHD.
Read more »
 FDA approves anti-obesity drug as first sleep apnea medicationPolitical News and Conservative Analysis About Congress, the President, and the Federal Government
FDA approves anti-obesity drug as first sleep apnea medicationPolitical News and Conservative Analysis About Congress, the President, and the Federal Government
Read more »
 FDA Approves First Drug for Obstructive Sleep ApneaThe FDA has approved Zepbound, a new drug for treating obstructive sleep apnea (OSA) in moderately to severely obese adults. Zepbound is intended to be used alongside lifestyle changes like diet and exercise.
FDA Approves First Drug for Obstructive Sleep ApneaThe FDA has approved Zepbound, a new drug for treating obstructive sleep apnea (OSA) in moderately to severely obese adults. Zepbound is intended to be used alongside lifestyle changes like diet and exercise.
Read more »
 FDA Approves First Drug for Sleep Apnea TreatmentThe US Food and Drug Administration (FDA) has granted approval for Zepbound, a weight-loss medication, to treat moderate to severe sleep apnea in obese patients. This marks a significant advancement in the treatment of sleep apnea, a condition affecting millions of Americans.
FDA Approves First Drug for Sleep Apnea TreatmentThe US Food and Drug Administration (FDA) has granted approval for Zepbound, a weight-loss medication, to treat moderate to severe sleep apnea in obese patients. This marks a significant advancement in the treatment of sleep apnea, a condition affecting millions of Americans.
Read more »
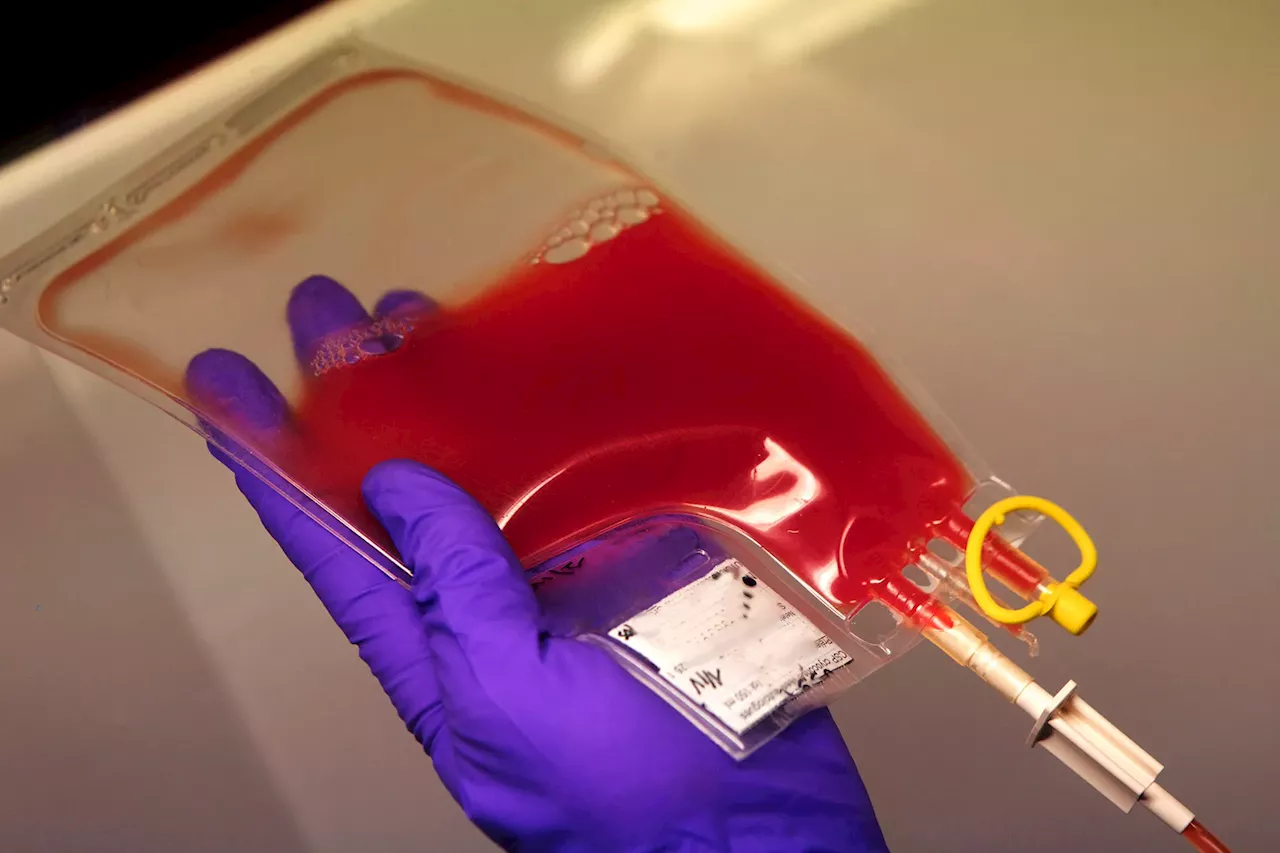 FDA Approves First Stem Cell Therapy for Life-Threatening Condition After Bone Marrow TransplantsThe U.S. Food and Drug Administration (FDA) has approved Remestemcel-L (Ryoncil), the first stem cell therapy for severe acute graft-versus-host disease (SR-aGVHD), a serious complication that can occur after bone marrow transplantation. SR-aGVHD affects about 25% of the 1,500 children in the U.S. who undergo bone marrow transplants annually. Ryoncil is derived from donor bone marrow stem cells and helps control the immune system's response by reducing inflammation and boosting the body's natural anti-inflammatory processes. The drug is also the first FDA-approved treatment for children ages 2 months and older with SR-aGVHD.
FDA Approves First Stem Cell Therapy for Life-Threatening Condition After Bone Marrow TransplantsThe U.S. Food and Drug Administration (FDA) has approved Remestemcel-L (Ryoncil), the first stem cell therapy for severe acute graft-versus-host disease (SR-aGVHD), a serious complication that can occur after bone marrow transplantation. SR-aGVHD affects about 25% of the 1,500 children in the U.S. who undergo bone marrow transplants annually. Ryoncil is derived from donor bone marrow stem cells and helps control the immune system's response by reducing inflammation and boosting the body's natural anti-inflammatory processes. The drug is also the first FDA-approved treatment for children ages 2 months and older with SR-aGVHD.
Read more »
