The FDA announced it has approved a new kind of drug to treat moderate to severe hot flashes caused by menopause.
For decades, hormone replacement therapy has been the treatmentBut use of those treatments fell sharply after results from the government-backed
in 2002 suggested taking a combination of estrogen and progestin might raise the risk of worrying conditions like breast cancer and heart disease. , though they say evidence shows that the benefits still outweigh the risks for many women struggling with symptoms. An antidepressant, paroxetine, is the main non-hormonal treatment alternative available for women. But its approval wasWhile true head-to-head studies have not been published comparing fezolinetant against menopausal hormone therapy, or MHT, research so far on the drug suggests it could be a promising alternative.
"Over the short-term, the safety and tolerability of fezolinetant and MHT appear comparable. However, longer term use of MHT carries serious increased risks," wrote the Institute for Clinical and Economic Review in a report
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Approves First Drug to Treat Alzheimer's AgitationThe antipsychotic brexpiprazole (Rexulti) is the first FDA-approved treatment option for agitation associated with Alzheimer's disease.
FDA Approves First Drug to Treat Alzheimer's AgitationThe antipsychotic brexpiprazole (Rexulti) is the first FDA-approved treatment option for agitation associated with Alzheimer's disease.
Read more »
 FDA approves nonhormonadrug to treat hot flashes and night sweatsThe pills will be marketed as Veozah to treat moderate to severe hot flashes, the most common symptom caused by menopause which can last for several years or as long as a decade or more.
FDA approves nonhormonadrug to treat hot flashes and night sweatsThe pills will be marketed as Veozah to treat moderate to severe hot flashes, the most common symptom caused by menopause which can last for several years or as long as a decade or more.
Read more »
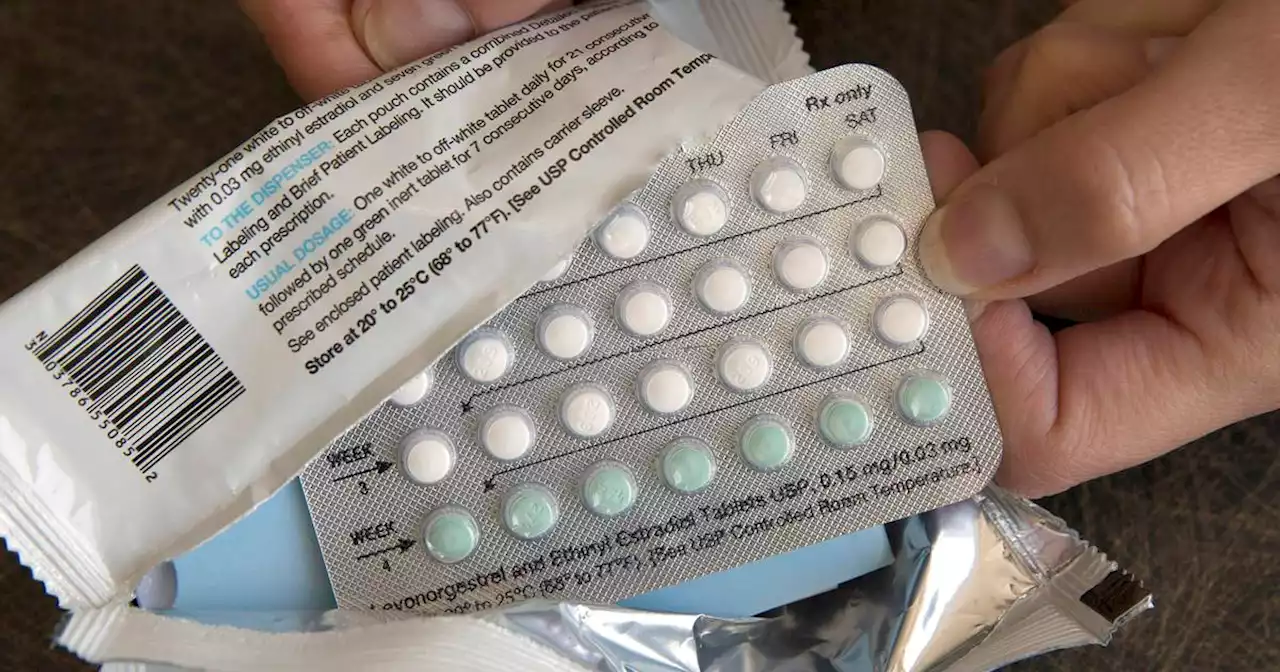 FDA panel backs over-the-counter sales of birth control pillFederal health advisers said that a decades-old birth control pill should be sold without a prescription, paving the way for a likely U.S. approval of the first over-the-counter contraceptive medication.
FDA panel backs over-the-counter sales of birth control pillFederal health advisers said that a decades-old birth control pill should be sold without a prescription, paving the way for a likely U.S. approval of the first over-the-counter contraceptive medication.
Read more »
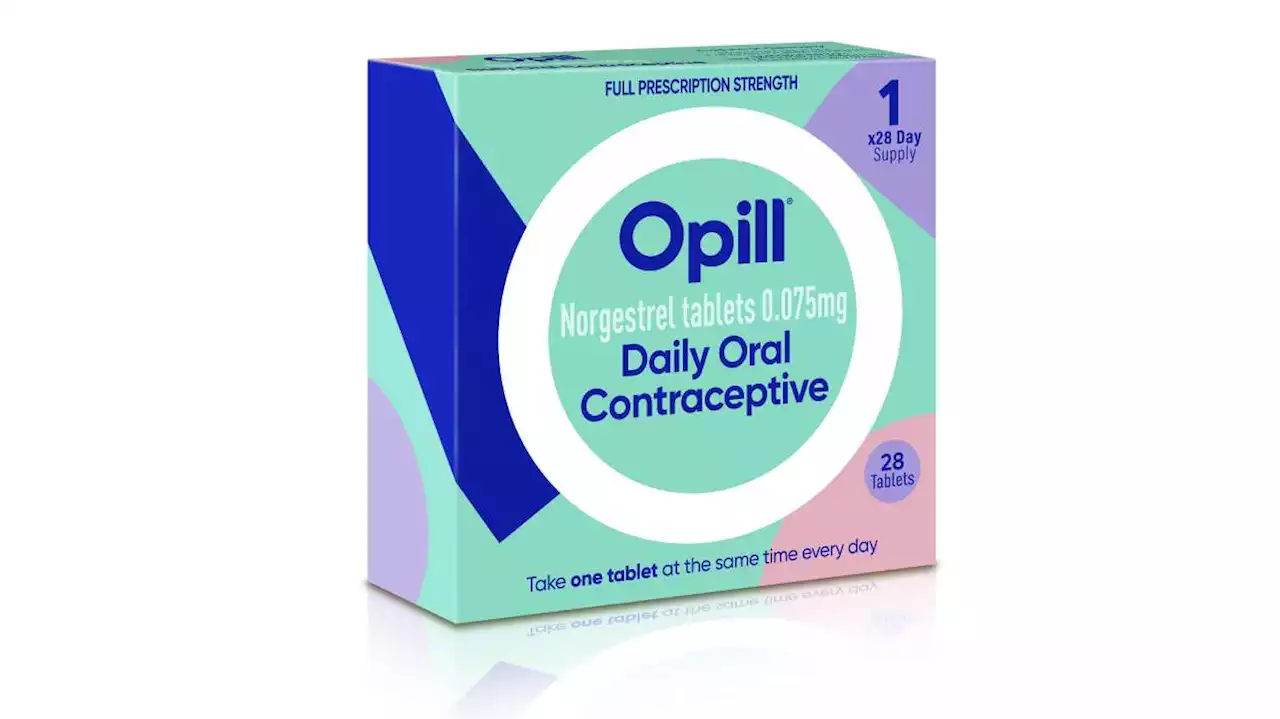 FDA panel backs over-the-counter sale of birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The unanimous vote on Wednesday by an FDA panel paves the way for what could be the 1st birth control pill available over the counter.
FDA panel backs over-the-counter sale of birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The unanimous vote on Wednesday by an FDA panel paves the way for what could be the 1st birth control pill available over the counter.
Read more »
 Vermont's new law grants strong abortion medication protectionsVermont's new abortion protections would keep mifepristone available even if FDA approval is later withdrawn.
Vermont's new law grants strong abortion medication protectionsVermont's new abortion protections would keep mifepristone available even if FDA approval is later withdrawn.
Read more »
