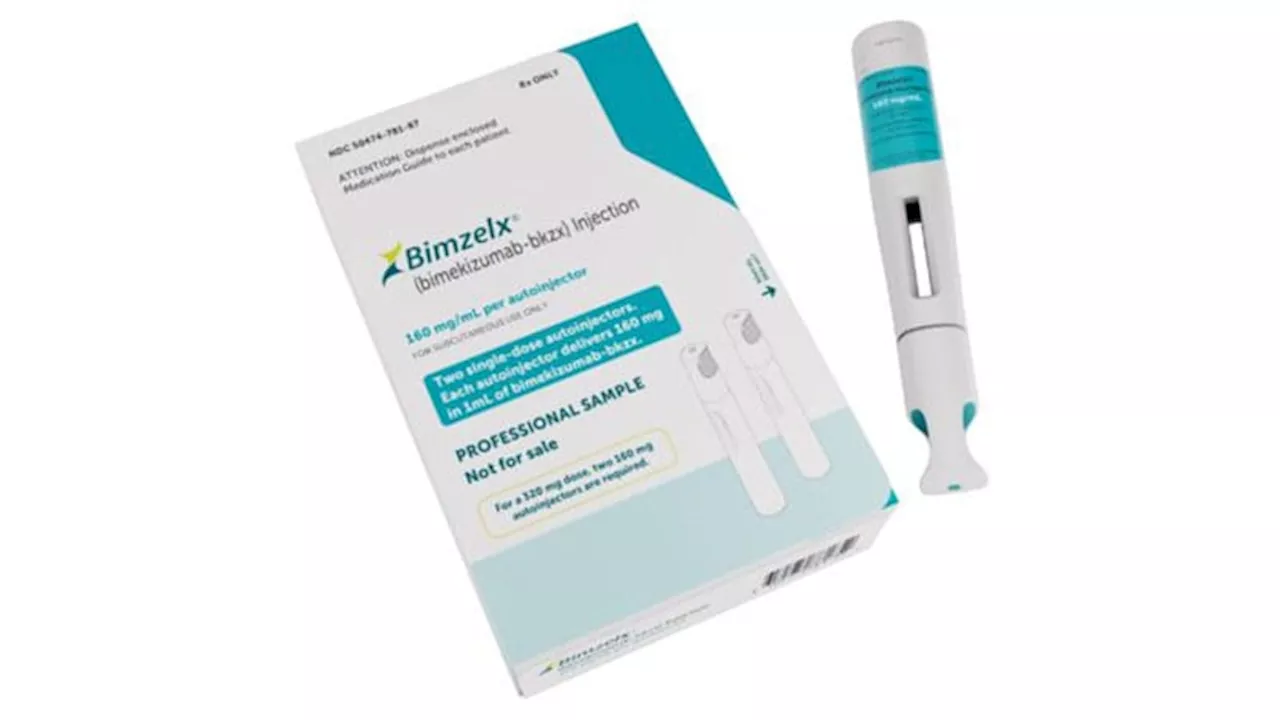
The drug is the first and only dual IL-17A and IL-17F inhibitor approved to treat four chronic immune-mediated inflammatory diseases.
"In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years," said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter.
In AS patients, 44.8% in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% receiving placebo. At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1. In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia,, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Arthropathic Psoriasis Psoriatic Arthropathy Psa Otolaryngology ENT Specialty Head And Neck Surgery ENT Speciality Psoriasis Upper Respiratory Infection Upper Respiratory Tract Infection Clinical Research Clinical Trials Clinical Studies Pre-Clinical Trial Double-Blind Study Double-Blind Studies Single-Blind Study Single-Blind Studies Plaque Psoriasis
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves first at-home syphilis test to combat U.S. rise in sexually transmitted infectionThe at-home syphilis test, which is available without a prescription, gives results in about 15 minutes. Other at-home syphilis tests currently on the market require consumers to send their sample to a lab, the FDA said.
FDA approves first at-home syphilis test to combat U.S. rise in sexually transmitted infectionThe at-home syphilis test, which is available without a prescription, gives results in about 15 minutes. Other at-home syphilis tests currently on the market require consumers to send their sample to a lab, the FDA said.
Read more »
 FDA Approves Apple's AirPods Pro as First Over-the-Counter Hearing AidsJust days after announcing a new hearing aid feature, Apple has received FDA authorization for the software powering this functionality. The FDA describes it as the first over-the-counter (OTC) hearing aid software device.
FDA Approves Apple's AirPods Pro as First Over-the-Counter Hearing AidsJust days after announcing a new hearing aid feature, Apple has received FDA authorization for the software powering this functionality. The FDA describes it as the first over-the-counter (OTC) hearing aid software device.
Read more »
 FDA Approves Apple AirPods Pro as Hearing Aids in Industry FirstThe Best in Science News and Amazing Breakthroughs
FDA Approves Apple AirPods Pro as Hearing Aids in Industry FirstThe Best in Science News and Amazing Breakthroughs
Read more »
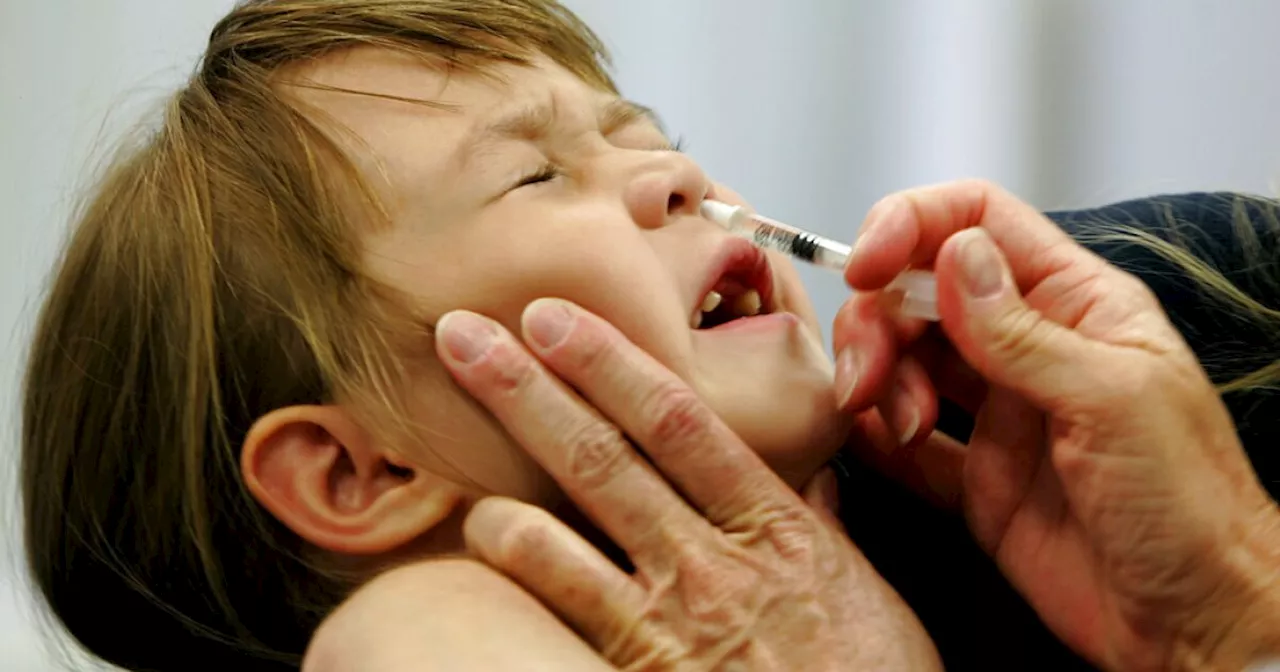 Doctor not included: FDA approves first flu vaccine that can be taken at homeThe Food and Drug Administration announced Friday it has approved the first flu vaccine that individuals can give to themselves at home, without the need for a healthcare professional.
Doctor not included: FDA approves first flu vaccine that can be taken at homeThe Food and Drug Administration announced Friday it has approved the first flu vaccine that individuals can give to themselves at home, without the need for a healthcare professional.
Read more »
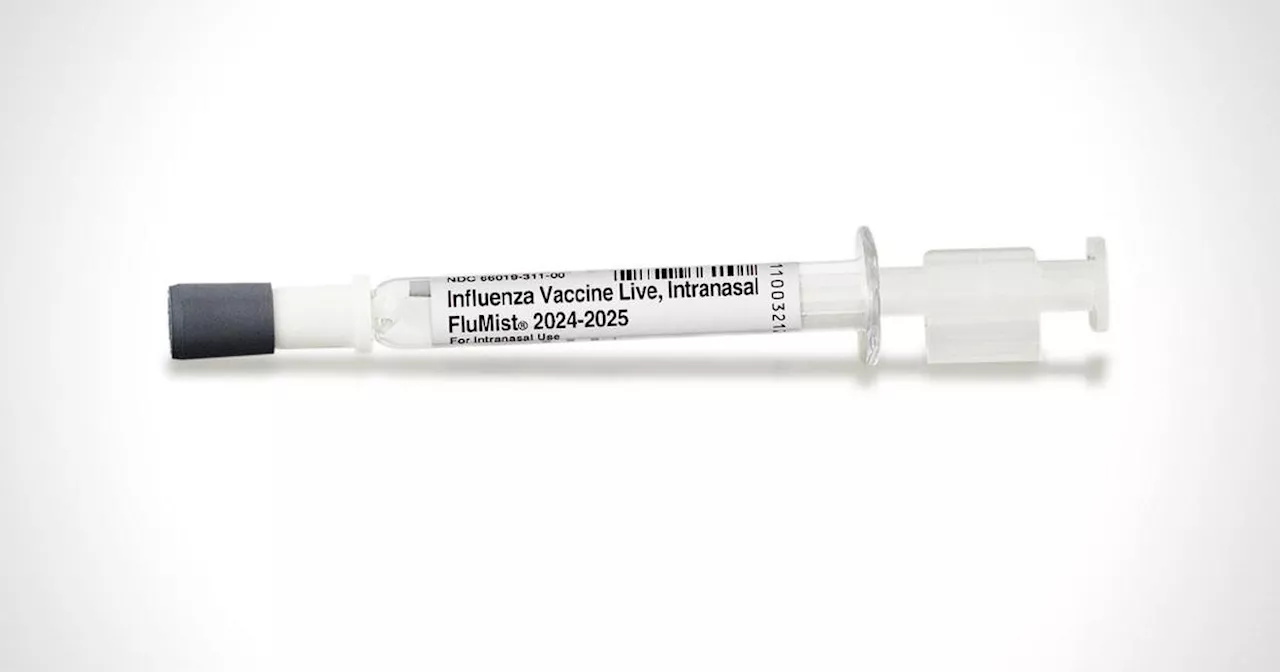 FDA approves first self-administered flu vaccine sprayAstraZeneca says the spray could be available 'as soon as next flu season.'
FDA approves first self-administered flu vaccine sprayAstraZeneca says the spray could be available 'as soon as next flu season.'
Read more »
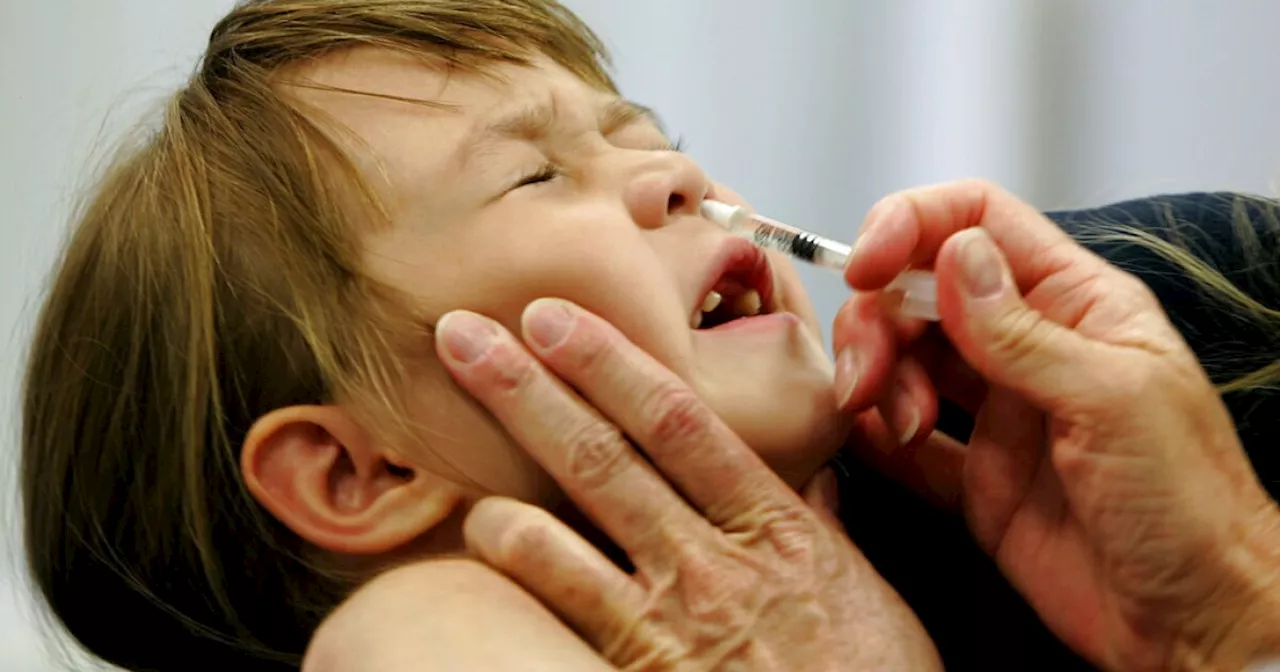 FDA approves first self-administered flu vaccineThe Food and Drug Administration (FDA) has approved FluMist, a nasal spray flu vaccine, allowing individuals to administer it at home without a healthcare professional. Users complete a screening and eligibility assessment through an online pharmacy, receive the vaccine by mail with instructions, and can choose to have it administered by a caregiver for children.
FDA approves first self-administered flu vaccineThe Food and Drug Administration (FDA) has approved FluMist, a nasal spray flu vaccine, allowing individuals to administer it at home without a healthcare professional. Users complete a screening and eligibility assessment through an online pharmacy, receive the vaccine by mail with instructions, and can choose to have it administered by a caregiver for children.
Read more »