The U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
This vaccine stimulates the immune system to make antibodies against a protein from the virus called the F protein that RSV uses to attach to human cells and cause infection.
This isn't the only vaccine that works in this manner. A booster of the tetanus, diphtheria, and pertussis vaccine, called Tdap, is also recommended to be given to pregnant women between 27- and 36-weeks' gestation to allow protective antibodies to pass on to the baby before birth. The FDA also said it's requiring Pfizer to conduct post-marketing studies "to assess the signal of serious risk of preterm birth and to assess hypertensive disorders of pregnancy, including pre-eclampsia."
RSV typically causes a mild illness for most people but can be dangerous for the elderly, infants and babies, with some vulnerable children at an even greater risk of severe illness, including those born prematurely, immunocompromised children and those suffering from congenital heart and lung diseases, the CDC said.can lead to difficulty breathing like fast breathing, and sometimes the infants have trouble feeding because they're breathing so hard.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
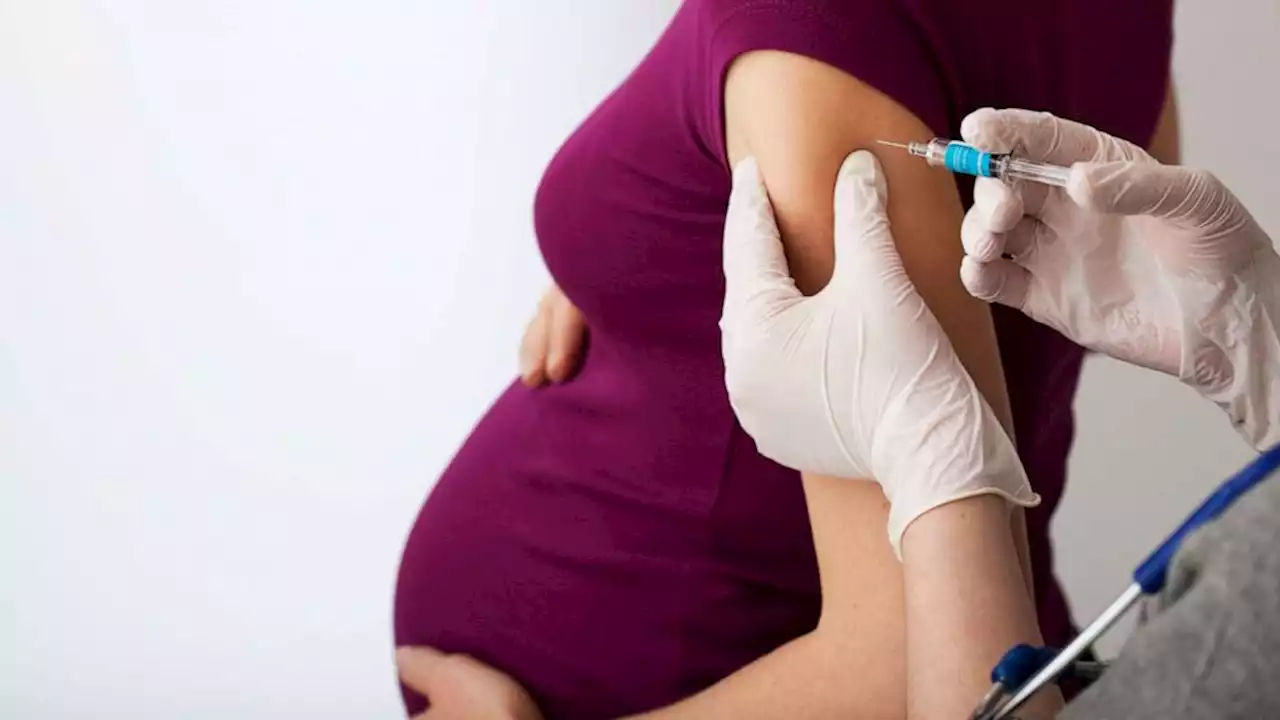 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Read more »
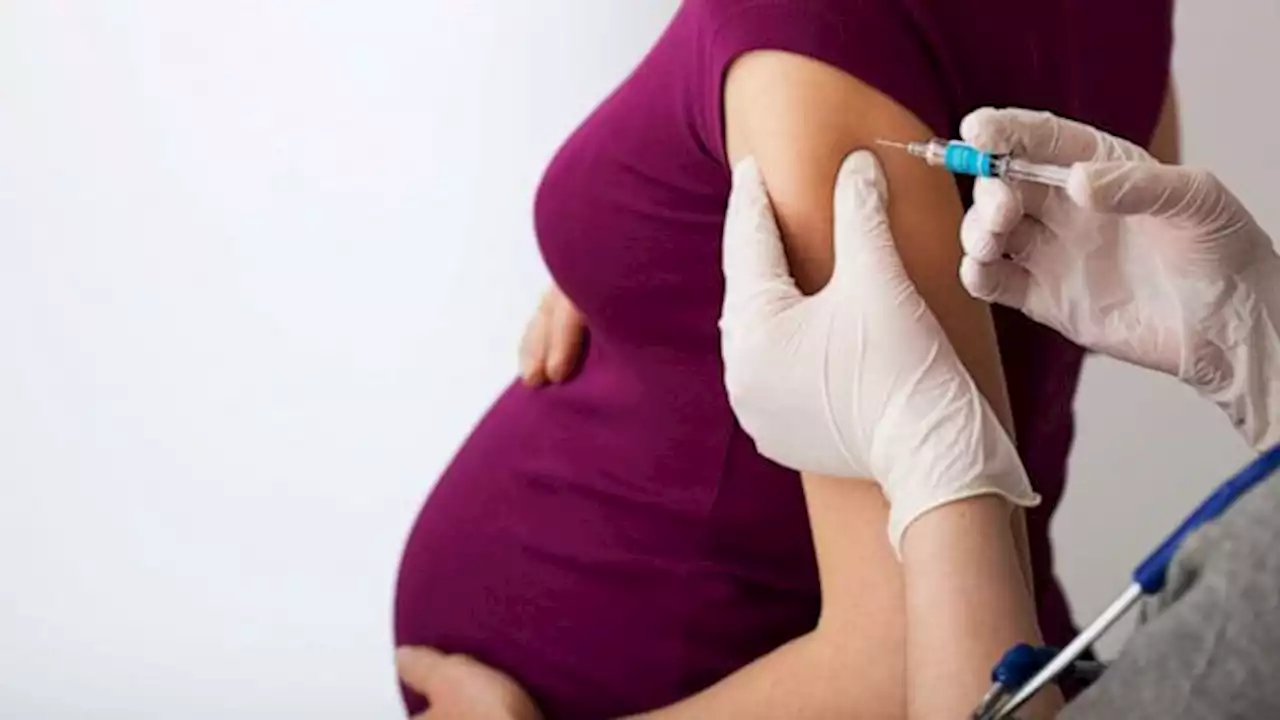 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Read more »
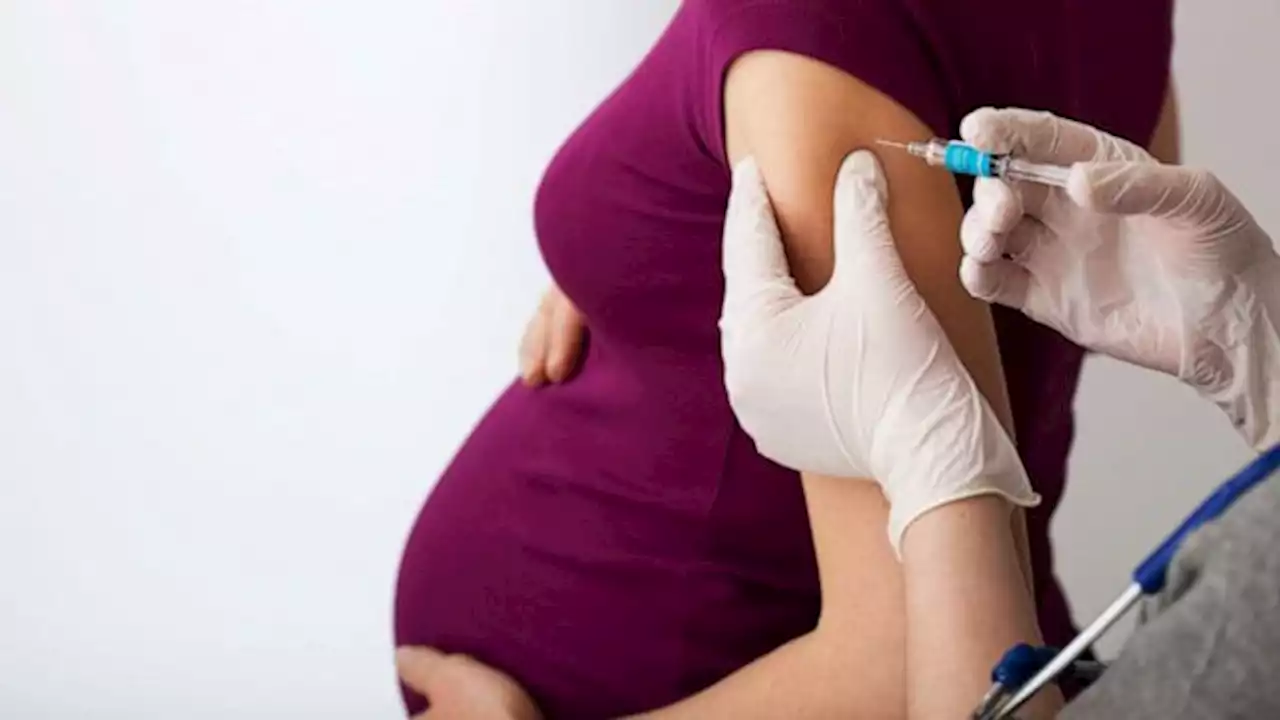 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Read more »
 US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
Read more »
 FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
Read more »