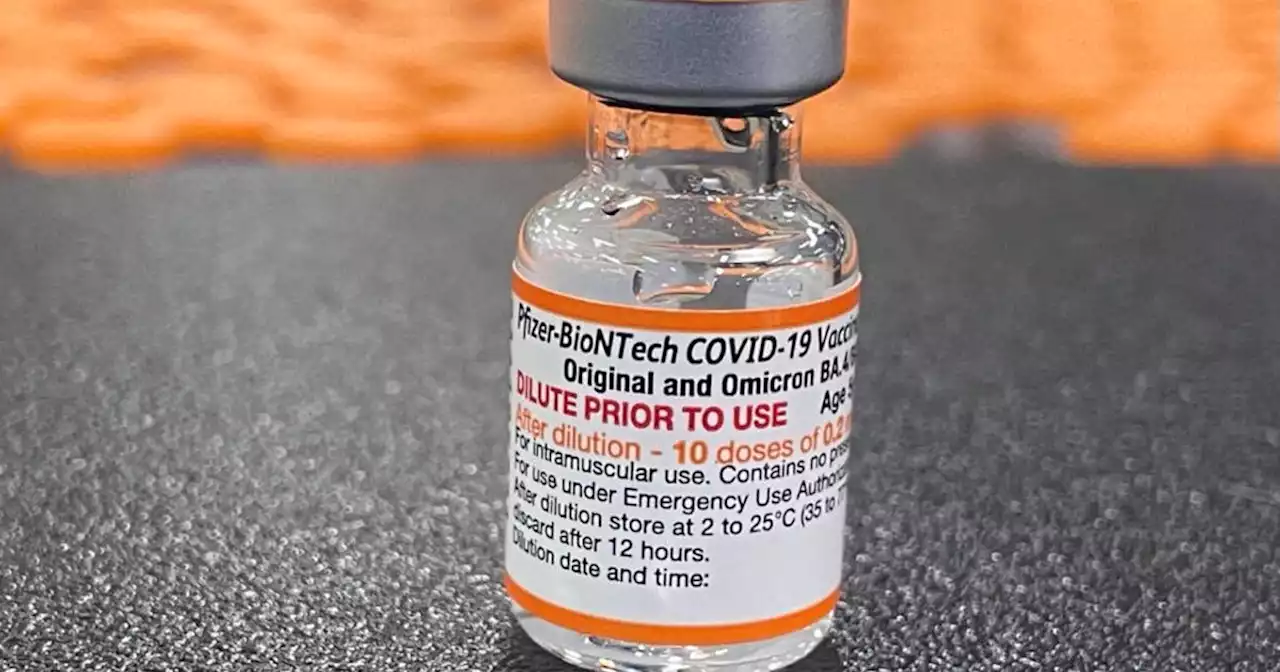
The Food and Drug Administration announced Wednesday that it had granted emergency use authorization for updated COVID-19 vaccine boosters for Americans as young as 5 years old.
"FDA's authorization of updated COVID-19 vaccines for this younger age group, and CDC's recommendation for use, are critical next steps forward in our country's vaccination program—a program that has helped provide increased protection against severe COVID-19 disease and death," CDC Director Dr. Rochelle Walensky said in a statement.
These new bivalent shots are manufactured in a nearly identical process from Pfizer-BioNTech and Moderna, aside from adding in a component designed to target the BA.4 and BA.5 variants. A spokesperson for the Department of Health and Human Services did not respond to a request for comment on how many shots were requested of the new Pfizer vaccines for younger ages. of the same formulation that is already being distributed for older age groups, and did not need to be pre-ordered.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 US clears updated COVID boosters for kids as young as 5Kids as young as 5 can soon get updated COVID-19 booster shots
US clears updated COVID boosters for kids as young as 5Kids as young as 5 can soon get updated COVID-19 booster shots
Read more »
 FDA clears updated COVID boosters for kids as young as 5There’s still one more step before kids get the new shot: The CDC must sign off on it.
FDA clears updated COVID boosters for kids as young as 5There’s still one more step before kids get the new shot: The CDC must sign off on it.
Read more »
 FDA clears updated COVID boosters for kids as young as 5The U.S. on Wednesday authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave.
FDA clears updated COVID boosters for kids as young as 5The U.S. on Wednesday authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave.
Read more »
 FDA clears updated COVID boosters for kids as young as 5Officials hope to expand protection against an expected winter surge.
FDA clears updated COVID boosters for kids as young as 5Officials hope to expand protection against an expected winter surge.
Read more »
 FDA clears updated COVID boosters for kids as young as 5The U.S. is about to offer updated COVID-19 booster shots to kids as young as 5
FDA clears updated COVID boosters for kids as young as 5The U.S. is about to offer updated COVID-19 booster shots to kids as young as 5
Read more »