Children as young as 5 are now eligible to receive the new updated COVID-19 boosters, which target the Omicron variant of the coronavirus. Both the FDA and the CDC signed off on the shots for this age group.
, which target the Omicron variant of the coronavirus. Both the Food Drug and Administration and the Centers for Disease Control and Prevention signed off on the shots for this age group on Wednesday.
The approved booster is what’s known as a bivalent vaccine, which can protect from more than one virus or subtype of a virus. This vaccine is designed to target both the original strain and the BA.4 and BA.5 Omicron subvariants. The BA.5 subvariant continues to dominate infections, accounting for about 80% of cases in the United States, according to theThe FDA authorized Pfizer-BioNTech's bivalent vaccine for kids ages 5 to 11.
After Wednesday’s authorization, the monovalent COVID-19 vaccines developed by Moderna and Pfizer, which target the original strain of the coronavirus only, will no longer be authorized as a booster shot for these age groups, the FDA said. But the monovalent vaccine will “continue to be authorized for primary series administration in individuals six months of age and older,” the agency noted in Wednesday’s statement.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Covid-19 Boosters Targeting Omicron Authorized for Kids as Young as 5The FDA’s decision broadens a campaign by health officials to bolster protection against the Omicron strains of the coronavirus.
Covid-19 Boosters Targeting Omicron Authorized for Kids as Young as 5The FDA’s decision broadens a campaign by health officials to bolster protection against the Omicron strains of the coronavirus.
Read more »
 Omicron-Targeting Covid-19 Boosters Recommended for Kids as Young as 5The FDA’s decision broadens a campaign by health officials to bolster protection against the Omicron strains of the coronavirus.
Omicron-Targeting Covid-19 Boosters Recommended for Kids as Young as 5The FDA’s decision broadens a campaign by health officials to bolster protection against the Omicron strains of the coronavirus.
Read more »
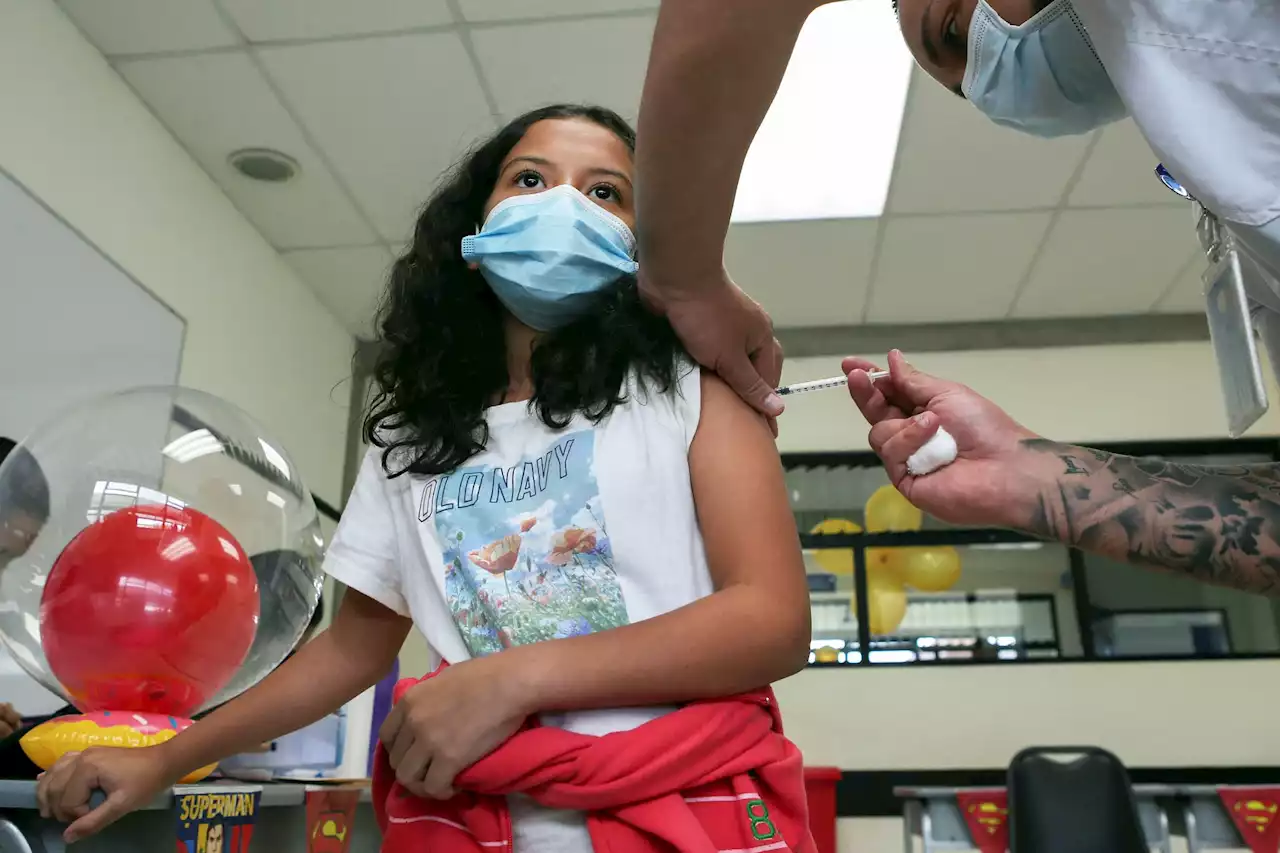 CDC Recommends Covid Omicron Booster Shots for Kids as Young as 5 Years OldChildren ages 5 to 11 are eligible for Pfizer's omicron shots and kids ages 6 through 17 are eligible for Moderna's shots two months after receiving their primary series or previous booster.
CDC Recommends Covid Omicron Booster Shots for Kids as Young as 5 Years OldChildren ages 5 to 11 are eligible for Pfizer's omicron shots and kids ages 6 through 17 are eligible for Moderna's shots two months after receiving their primary series or previous booster.
Read more »
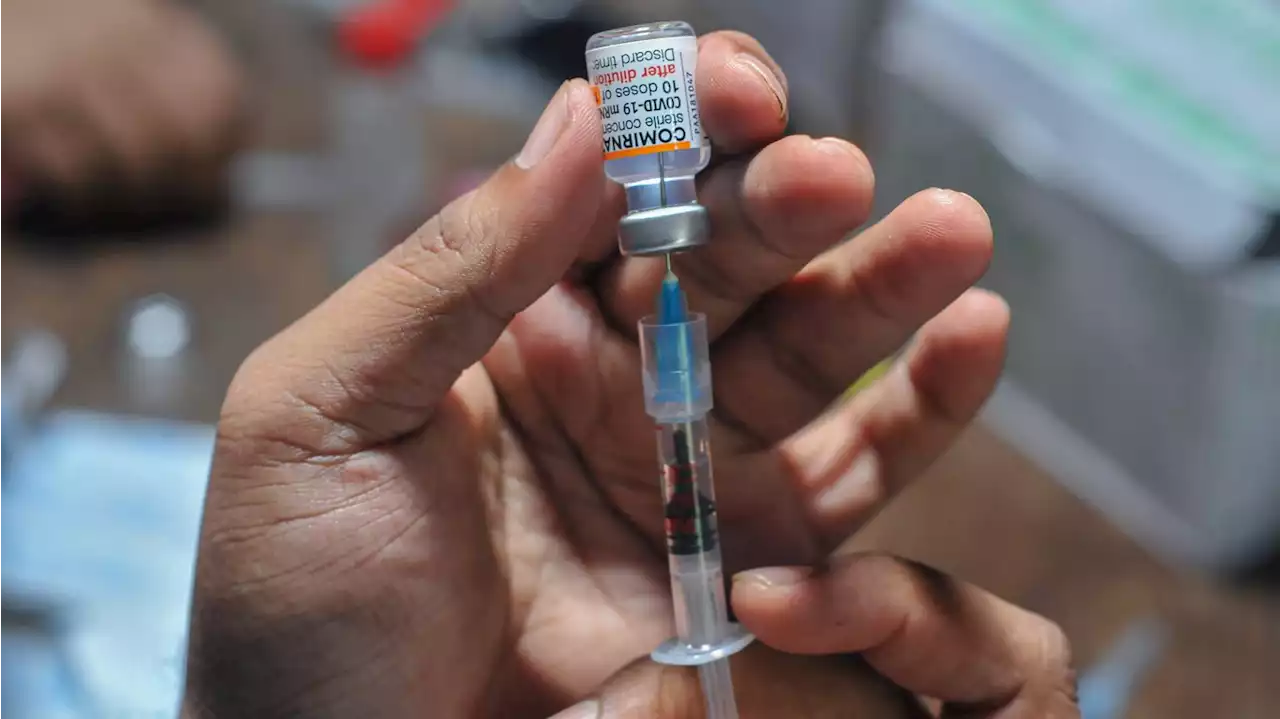 FDA authorizes COVID-19 Omicron booster for kidsThe booster shots are now authorized for children as young as 5 years old.
FDA authorizes COVID-19 Omicron booster for kidsThe booster shots are now authorized for children as young as 5 years old.
Read more »
 FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
Read more »
 Could New COVID Variants Spark Wave Similar to Omicron Last Winter?For the last few months, experts had expressed optimism that the BA.5 variant represented nearly all COVID cases as new COVID booster shots, specifically designed to target that variant, rolled out.
Could New COVID Variants Spark Wave Similar to Omicron Last Winter?For the last few months, experts had expressed optimism that the BA.5 variant represented nearly all COVID cases as new COVID booster shots, specifically designed to target that variant, rolled out.
Read more »
