This study introduces adipose manipulation transplantation (AMT), a groundbreaking therapeutic strategy that utilizes engineered adipocytes to starve tumors of essential nutrients. By enhancing the glucose and fatty acid uptake capabilities of white adipose tissue, AMT effectively outcompetes cancer cells for vital resources, hindering their growth and progression. Preclinical studies in various cancer models, including human breast cancer, demonstrate the significant efficacy of AMT in suppressing tumor size, angiogenesis, and hypoxia. Furthermore, the study explores the potential for customizing AMT by targeting specific metabolic pathways crucial for tumor survival.
Tumors exhibit an increased ability to obtain and metabolize nutrients. Here, we implant engineered adipocytes that outcompete tumors for nutrients and show that they can substantially reduce cancer progression, a technology termed adipose manipulation transplantation ( AMT ). Adipocytes engineered to use increased amounts of glucose and fatty acids by upregulating were placed alongside cancer cells or xenografts, leading to significant cancer suppression.
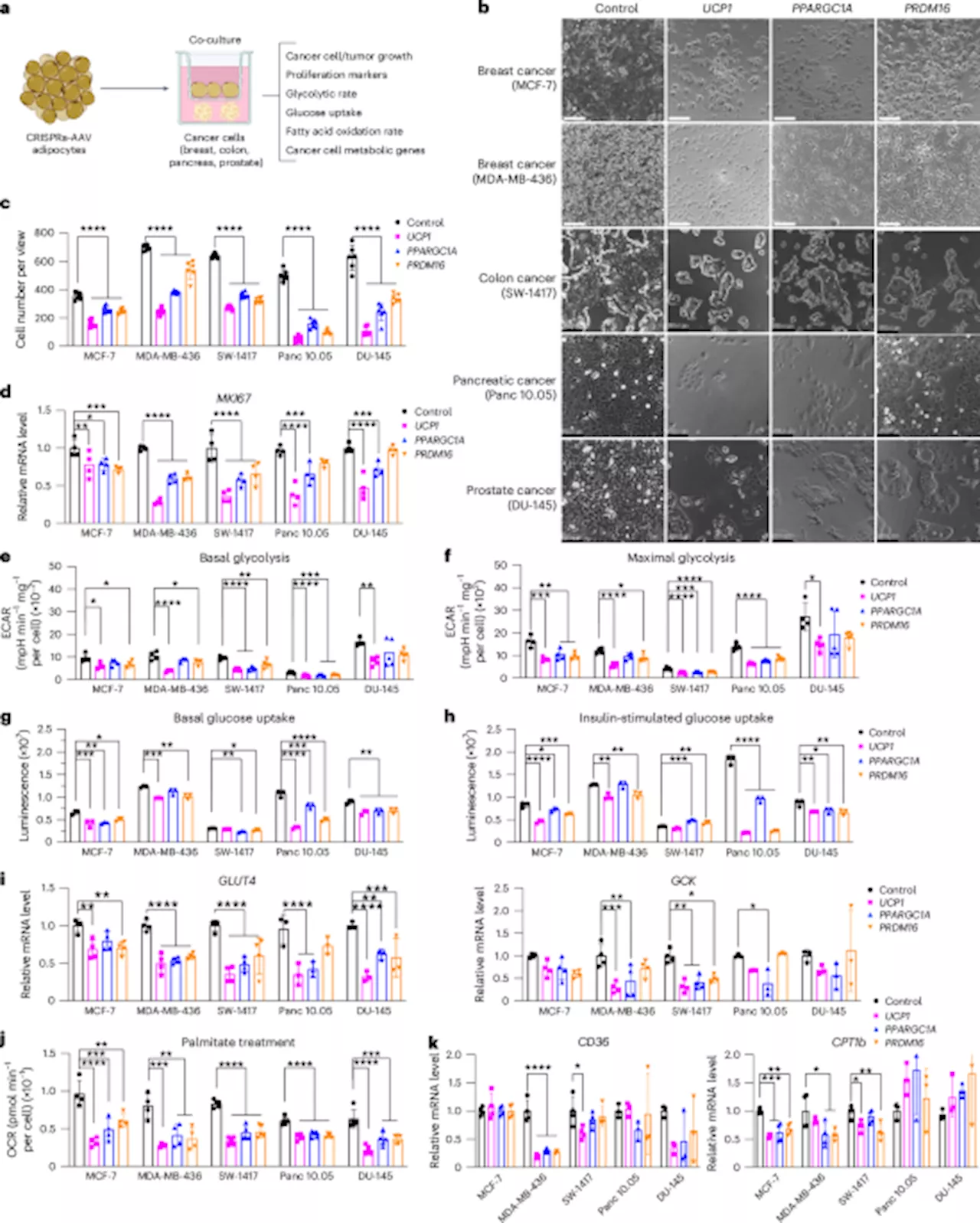
Transplanting modulated adipose organoids in pancreatic or breast cancer genetic mouse models suppressed their growth and decreased angiogenesis and hypoxia. Co-culturing patient-derived engineered adipocytes with tumor organoids from dissected human breast cancers significantly suppressed cancer progression and proliferation. In addition, cancer growth was impaired by inducing engineered adipose organoids to outcompete tumors using tetracycline or placing them in an integrated cell-scaffold delivery platform and implanting them next to the tumor. Finally, we show that upregulating in adipose organoids can outcompete a uridine-dependent pancreatic ductal adenocarcinoma for uridine and suppress its growth, demonstrating the potential customization of AMT. Tumors are complex tissues composed of cancerous and non-cancerous cells in a hypoxic and nutrient-deprived microenvironment. The tumor microenvironment contains heterogeneous cell populations, including immune cells, mesenchymal support cells and matrix components that contribute to tumor growth and progression. To survive this environment, tumors are capable of reprogramming metabolic pathways to better use available substrates in the surrounding tumor microenvironment, ultimately becoming dependent on these pathways for continued growth and survival. In contrast to normal cells, the main pathway of glucose metabolism in cancer cells is aerobic glycolysis, termed the Warburg effect. The increase in glycolytic flux allows glycolytic intermediates to supply subsidiary pathways to fulfil the metabolic demands of proliferating cells. During hypoxia, cancer cells also undergo metabolic reprogramming to increase lipid use, as fatty acids produce twice the energy of glucose. There have been many efforts to target cancer glucose and fatty acid metabolism for therapeutic purposes. For glycolysis, these include drugs that target hexokinase 2 (HK2), which is involved in the initial steps in glycolysis, using ATP from the mitochondria to phosphorylate glucose, or drugs that target glucose transporters (GLUT1 and GLUT4). These include drugs that target lipid uptake (targeting proteins such as LXR, CD36 and FABP4/5), lipogenic enzymes (such as ACC, ACLY, FASN and SCD1) and proteins involved in intracellular lipid homeostasis (such as CPT1A, PPARG and others). In addition, recent work has shown that cold activation of brown adipose tissue (BAT), which dissipates energy by non-shivering thermogenesis, increases adipocyte glucose uptake and lipid metabolism and significantly inhibits tumor growth. Here, we set out to develop a therapeutic approach, termed adipose manipulation transplantation (AMT), that uses two unique abilities of white adipose tissue (WAT). First, it can be readily extracted in the clinic by liposuction and implanted through reconstructive surgery; second, it can change into a BAT-like tissue, called browning or beiginginduces browning, subsequently increasing glucose and fat metabolism in human white adipocytes and adipose organoids. Co-culturing of these CRISPRa-modulated adipocytes with various cancer cell lines (breast, colon, pancreatic or prostate cancer) significantly suppresses cancer cell proliferation as well as decreases glucose uptake, glycolysis and fatty acid oxidation (FAO) capacity in the cancer cells. Subcutaneously co-transplanting CRISPRa-modulated human adipose organoids and cancer cell xenografts (two different breast cancer lines, pancreatic or prostate) into immune-compromised mice leads to significantly reduced tumor size with decreased hypoxia and angiogenesis. Implantation of engineered adipose organoids into pancreatic or breast cancer genetic mouse models significantly suppresses cancer progression. Furthermore, in the breast cancer model, we show that their implantation both near and distal to the tumor leads to similar results. To further demonstrate the therapeutic potential of this approach, we show that adipocytes isolated from resected human breast tissues can be similarly manipulated with CRISPRa and can inhibit the growth of patient-derived breast cancer organoids as well as the proliferation of high-risk non-cancerous breast tissues such as those from patients withmutation
Cancer Adipose Tissue AMT Metabolism Glucose Uptake Fatty Acids Therapy Preclinical Studies
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Researchers discover new way to customize living materials for tissue engineering, drug delivery and 3D printingResearchers have revealed novel sequence-structure-property relationships for customizing engineered living materials (ELMs), enabling more precise control over their structure and how they respond to deformation forces like stretching or compression.
Researchers discover new way to customize living materials for tissue engineering, drug delivery and 3D printingResearchers have revealed novel sequence-structure-property relationships for customizing engineered living materials (ELMs), enabling more precise control over their structure and how they respond to deformation forces like stretching or compression.
Read more »
 UCD School of Biosystems and Food Engineering Seeks Assistant Professor of Artificial IntelligenceThe UCD School of Biosystems and Food Engineering is searching for an Assistant Professor of Artificial Intelligence in Food Engineering to lead groundbreaking research and inspire students in this field. The ideal candidate will have strengths in areas like spectral imaging, sensor design, machine learning, and digital technologies for agriculture and food security. They will contribute to developing solutions for sustainable food systems engineering and biological systems engineering for the circular bioeconomy through local, national, and international engagement.
UCD School of Biosystems and Food Engineering Seeks Assistant Professor of Artificial IntelligenceThe UCD School of Biosystems and Food Engineering is searching for an Assistant Professor of Artificial Intelligence in Food Engineering to lead groundbreaking research and inspire students in this field. The ideal candidate will have strengths in areas like spectral imaging, sensor design, machine learning, and digital technologies for agriculture and food security. They will contribute to developing solutions for sustainable food systems engineering and biological systems engineering for the circular bioeconomy through local, national, and international engagement.
Read more »
 Brain tissue may have higher amounts of microplastics than other organs, study findsBrain samples contained 10 times more microplastics than other organs.
Brain tissue may have higher amounts of microplastics than other organs, study findsBrain samples contained 10 times more microplastics than other organs.
Read more »
 Microplastics and Nanoplastics Found in High Concentrations in Human Brain TissueA new study published in February 2023 reveals the alarming presence of microplastics and nanoplastics in human brain tissue, raising concerns about the potential health implications of these tiny plastic particles. Researchers found significantly higher concentrations of MNPs in brain tissue compared to other organs, challenging the notion that the blood-brain barrier effectively protects the brain from these pollutants. The study also highlights the unexpected shapes and types of plastic found in the brain, suggesting that the long-term effects of microplastic exposure remain largely unknown.
Microplastics and Nanoplastics Found in High Concentrations in Human Brain TissueA new study published in February 2023 reveals the alarming presence of microplastics and nanoplastics in human brain tissue, raising concerns about the potential health implications of these tiny plastic particles. Researchers found significantly higher concentrations of MNPs in brain tissue compared to other organs, challenging the notion that the blood-brain barrier effectively protects the brain from these pollutants. The study also highlights the unexpected shapes and types of plastic found in the brain, suggesting that the long-term effects of microplastic exposure remain largely unknown.
Read more »
 Brain Tissue Found to Contain 10 Times More Microplastics Than Other OrgansA new study reveals that brain tissue contains significantly higher levels of microplastics compared to other organs like the liver and kidneys. The research, which analyzed samples from 47 cadavers, found an average of 4,800 micrograms of microplastics per gram of brain tissue. While the study doesn't establish a direct link between microplastics and health issues, it raises concerns about the potential impact of plastic pollution on human health.
Brain Tissue Found to Contain 10 Times More Microplastics Than Other OrgansA new study reveals that brain tissue contains significantly higher levels of microplastics compared to other organs like the liver and kidneys. The research, which analyzed samples from 47 cadavers, found an average of 4,800 micrograms of microplastics per gram of brain tissue. While the study doesn't establish a direct link between microplastics and health issues, it raises concerns about the potential impact of plastic pollution on human health.
Read more »
 Plesiosaur Soft Tissue Analysis Reveals Unique Skin Adaptation for Swimming and Seabed NavigationFor the first time, researchers have analyzed soft tissue from a fossilized plesiosaur, revealing a unique combination of smooth and scaly skin that likely helped the ancient marine reptile both swim efficiently and maneuver rough seafloors.
Plesiosaur Soft Tissue Analysis Reveals Unique Skin Adaptation for Swimming and Seabed NavigationFor the first time, researchers have analyzed soft tissue from a fossilized plesiosaur, revealing a unique combination of smooth and scaly skin that likely helped the ancient marine reptile both swim efficiently and maneuver rough seafloors.
Read more »