Eli Lilly has intervened in a legal battle between compounding pharmacies and the U.S. Food and Drug Administration (FDA) regarding the availability of Lilly's weight-loss and diabetes drugs. The company argues that the FDA's determination that these drugs are no longer in short supply could threaten its interests.
Eli Lilly has asked to join in opposing a lawsuit brought by compounding pharmacies against the U.S. Food and Drug Administration over the agency's decision that Lilly's blockbuster weight-loss and diabetes drugs are no longer in short supply.
In a motion filed in Fort Worth, Texas federal court late on Wednesday, Lilly said it could not rely on the FDA to fully defend its interests in the case, which will determine whether compounding pharmacies and facilities can keep selling cheaper versions of the company's weight-loss drug Zepbound and diabetes medicine Mounjaro, which have the same active ingredient, tirzepatide. The FDA declined to comment. The Outsourcing Facilities Association, which brought the lawsuit along with a Texas compounding pharmacy did not immediately respond to requests for comment. The compounded drugs at issue, which are essentially copies of the branded prescription medicines but not approved by the FDA, can only be made in significant amounts if there is a shortage.In response to the lawsuit, the agency agreed to reconsider its decision but on Dec. 19 affirmed that there is no shortage. At the time, FDA said it would not take any enforcement action for at least 60 days, and the compounding industry is still seeking a court order reversing the agency's decision. Lilly said in Wednesday's motion that it needed to join the case to defend its own interests because it could not be sure that the FDA would appeal if the court ruled against it. Lilly also said it believed compounding pharmacies, as opposed to larger so-called outsourcing facilities, may not manufacture compounded drugs even if there is a shortage. It said that may be at odds with the FDA's view. Novo Nordisk's rival weight-loss drug Wegovy remains on the FDA's shortage list
Eli Lilly FDA Lawsuit Compounding Pharmacies Drug Shortages
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA says Eli Lilly's weight loss drug Zepbound is no longer in shortageThe decision that will eventually bar compounding pharmacies from making unbranded versions of the injection.
FDA says Eli Lilly's weight loss drug Zepbound is no longer in shortageThe decision that will eventually bar compounding pharmacies from making unbranded versions of the injection.
Read more »
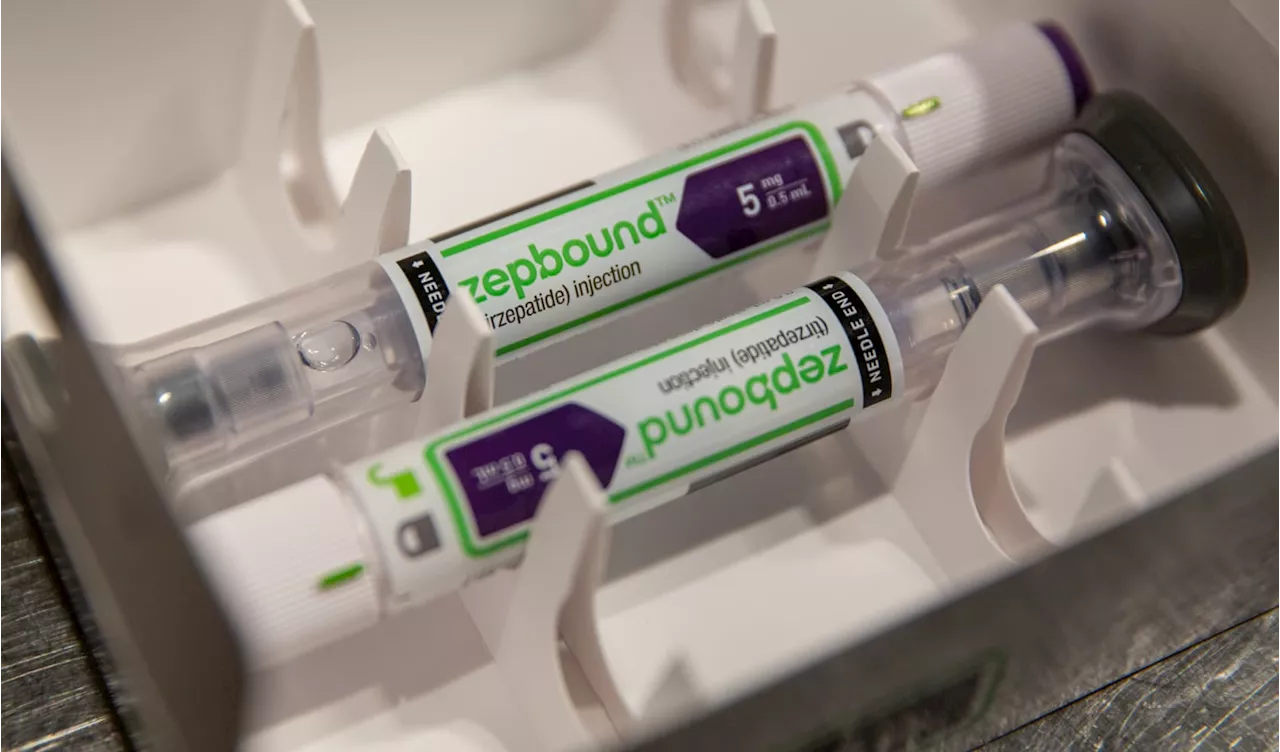 FDA approves Eli Lilly's weight loss drug Zepbound for sleep apnea, expanding use in U.S.The agency’s decision expands the use of Zepbound and could potentially pave the way for Eli Lilly to gain broader insurance coverage for the treatment.
FDA approves Eli Lilly's weight loss drug Zepbound for sleep apnea, expanding use in U.S.The agency’s decision expands the use of Zepbound and could potentially pave the way for Eli Lilly to gain broader insurance coverage for the treatment.
Read more »
 FDA approves Eli Lilly’s obesity medication for obstructive sleep apneaIn a study, people who took Zepbound had fewer breathing interruptions.
FDA approves Eli Lilly’s obesity medication for obstructive sleep apneaIn a study, people who took Zepbound had fewer breathing interruptions.
Read more »
 FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
Read more »
 FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
Read more »
 FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
FDA approves Eli Lilly's obesity medication for obstructive sleep apneaThe new approval means that insurance providers will likely cover the medication for people with sleep apnea and obesity.
Read more »
