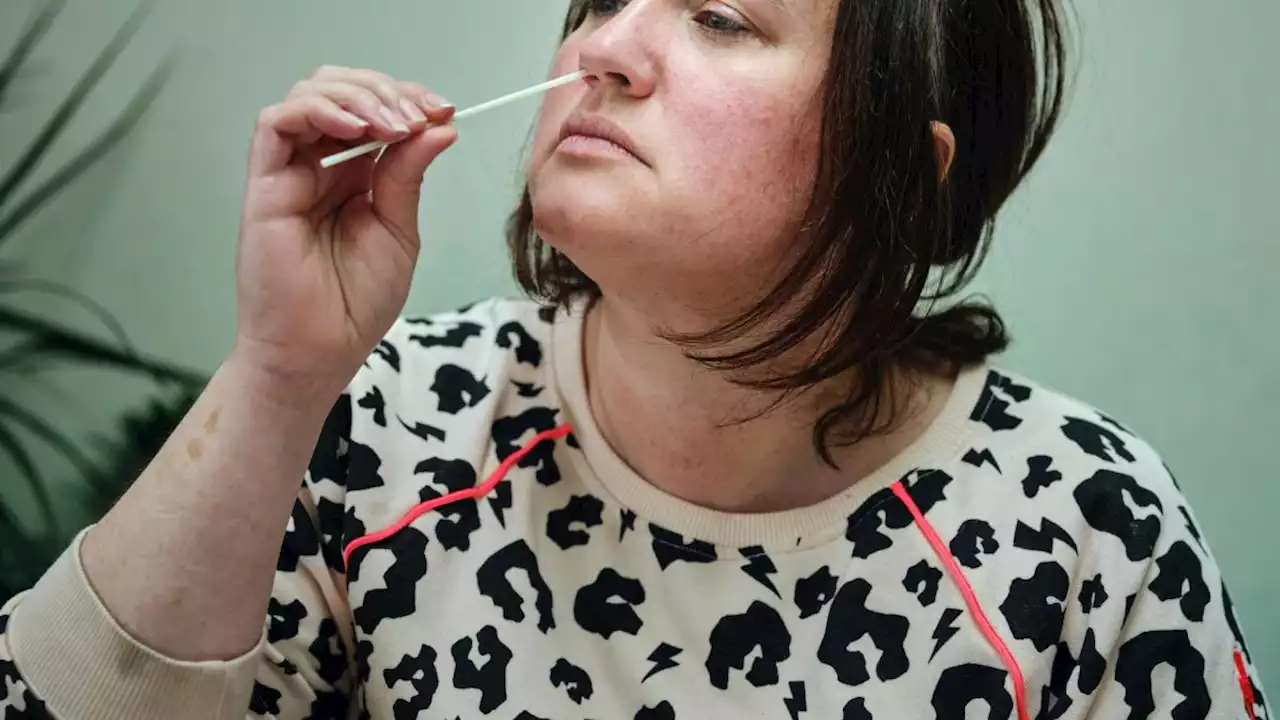
The Food and Drug Administration authorized a new test that detects SARS-CoV-2, influenza A and influenza B.
The U.S. Food and Drug Administration has authorized the first over-the-counter combination test for both influenza and COVID-19, the agency announced on Feb. 24. However, the test's maker recently filed for bankruptcy, so it's unclear whether the company has the resources to ramp up manufacturing and sell the test on a large scale, STAT reported .
The single-use, at-home test kit, made by the California-headquartered company Lucira Health, can differentiate between the two major types of influenza viruses — influenza A and influenza B — as well as detect SARS-CoV-2, the virus that causes COVID-19. The test uses nasal swab samples and provides results in 30 minutes or less; it's intended for use in individuals who've developed signs and symptoms of a respiratory tract infection.
"In individuals with symptoms, the Lucira COVID-19 & Flu Home Test correctly identified 99.3% of negative and 90% of positive Influenza A samples, 100% of negative and 88.3% of positive COVID-19 samples and 99.9% of negative Influenza B samples," the FDA statement reads. The agency noted that, since influenza B viruses are circulating at extremely low levels compared with influenza A,"there are currently not enough cases of Influenza B circulating to include in a clinical study." Because of this, Lucira confirmed that its test can detect influenza B by using"contrived" viruses, not taken directly from patients but rather grown in a lab setting.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes combination flu-COVID test for home useThe Food and Drug Administration has approved the first combination test for flu and COVID-19 that can be used at home, giving consumers an easy way to determine if a runny nose is caused by either disease.
FDA authorizes combination flu-COVID test for home useThe Food and Drug Administration has approved the first combination test for flu and COVID-19 that can be used at home, giving consumers an easy way to determine if a runny nose is caused by either disease.
Read more »
 'Potential lab incident': FBI director Wray speaks publicly for 1st time on COVID originFBI director Christopher Wray spoke publicly for the first time on the bureau's assessment that the COVID-19 virus 'most likely' originated from a potential lab incident in Wuhan, China.
'Potential lab incident': FBI director Wray speaks publicly for 1st time on COVID originFBI director Christopher Wray spoke publicly for the first time on the bureau's assessment that the COVID-19 virus 'most likely' originated from a potential lab incident in Wuhan, China.
Read more »
 'Potential lab incident': FBI director Wray speaks publicly for 1st time on COVID-19 originFBI director Christopher Wray spoke publicly for the first time on the bureau's assessment that the COVID-19 virus 'most likely' originated from a potential lab incident in Wuhan, China.
'Potential lab incident': FBI director Wray speaks publicly for 1st time on COVID-19 originFBI director Christopher Wray spoke publicly for the first time on the bureau's assessment that the COVID-19 virus 'most likely' originated from a potential lab incident in Wuhan, China.
Read more »